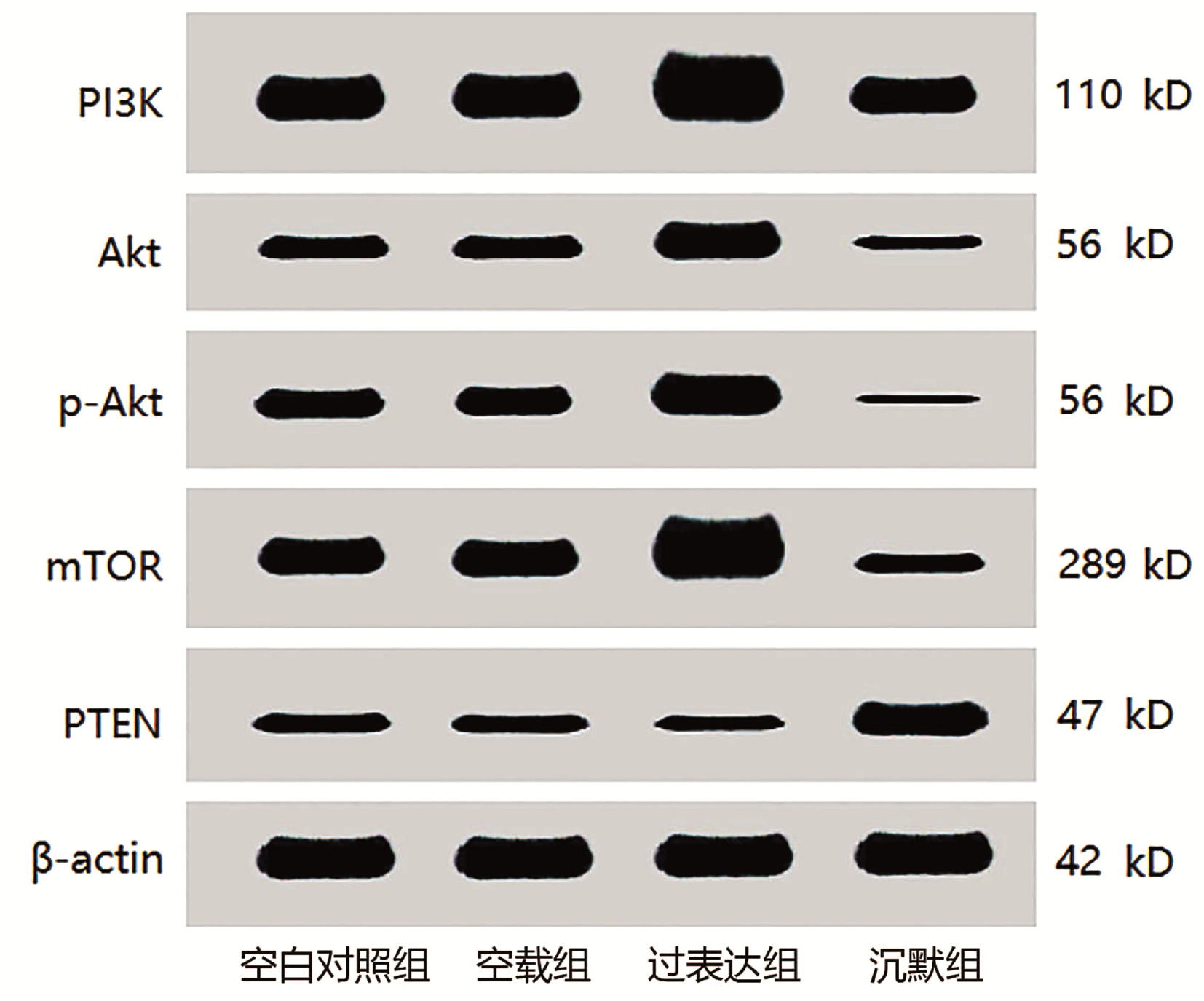
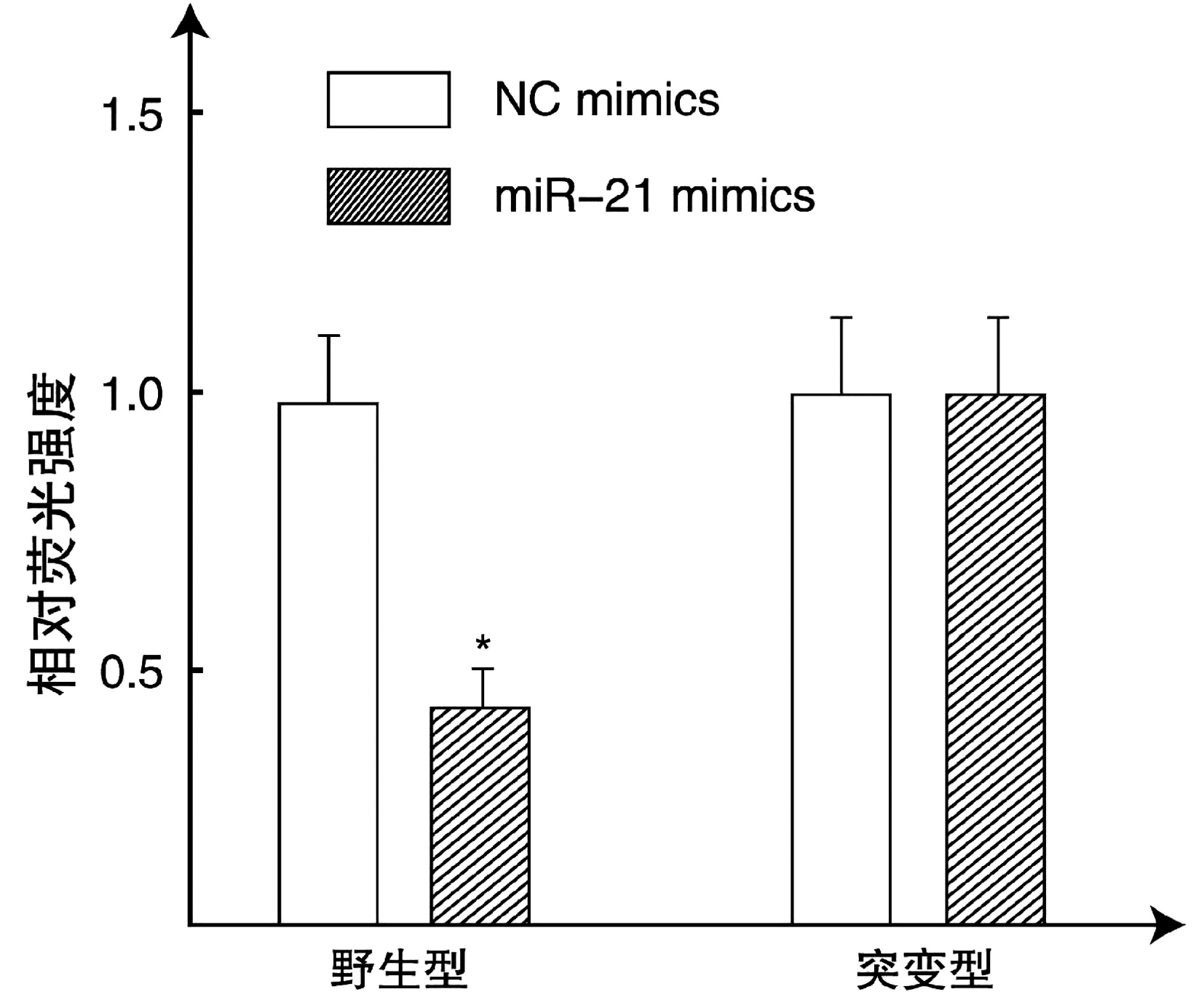
| [1] |
CARPINO G, CARDINALE V, FOLSERAAS T, et al. Neoplastic transformation of the peribiliary stem cell niche in cholangiocarcinoma arisen in primary sclerosing cholangitis[J]. Hepatology, 2019, 69(2): 622-638. DOI: 10.1002/hep.30210. |
| [2] |
KHAN SA, TAVOLARI S, BRANDI G. Cholangiocarcinoma: Epidemiology and risk factors[J]. Liver Int, 2019, 39(Suppl 1): 19-31. DOI: 10.1111/liv.14095. |
| [3] |
WANG M, CHEN Z, GUO P, et al. Therapy for advanced cholangiocarcinoma: Current knowledge and future potential[J]. J Cell Mol Med, 2021, 25(2): 618-628. DOI: 10.1111/jcmm.16151. |
| [4] |
CILLO U, FONDEVILA C, DONADON M, et al. Surgery for cholangiocarcinoma[J]. Liver Int, 2019, 39 (Suppl 1): 143-155. DOI: 10.1111/liv.14089. |
| [5] |
KONTOMANOLIS EN, KOUTRAS A, SYLLAIOS A, et al. Role of oncogenes and tumor-suppressor genes in carcinogenesis: A review[J]. Anticancer Res, 2020, 40(11): 6009-6015. DOI: 10.21873/anticanres.14622. |
| [6] |
CARNEIRO BA, EL-DEIRY WS. Targeting apoptosis in cancer therapy[J]. Nat Rev Clin Oncol, 2020, 17(7): 395-417. DOI: 10.1038/s41571-020-0341-y. |
| [7] |
JIANG N, DAI Q, SU X, et al. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior[J]. Mol Biol Rep, 2020, 47(6): 4587-4629. DOI: 10.1007/s11033-020-05435-1. |
| [8] |
ZHU B, WEI Y. Antitumor activity of celastrol by inhibition of proliferation, invasion, and migration in cholangiocarcinoma via PTEN/PI3K/Akt pathway[J]. Cancer Med, 2020, 9(2): 783-796. DOI: 10.1002/cam4.2719. |
| [9] |
CHEN L, YANG ZS, ZHOU YZ, et al. Dihydromyricetin inhibits cell proliferation, migration, invasion and promotes apoptosis via regulating miR-21 in human cholangiocarcinoma cells[J]. J Cancer, 2020, 11(19): 5689-5699. DOI: 10.7150/jca.45970. |
| [10] |
LIU H, WANG J, TAO Y, et al. Curcumol inhibits colorectal cancer proliferation by targeting miR-21 and modulated PTEN/PI3K/Akt pathways[J]. Life Sci, 2019, 221: 354-361. DOI: 10.1016/j.lfs.2019.02.049. |
| [11] |
SCHMITTGEN TD, LIVAK KJ. Analyzing real-time PCR data by the comparative C(T) method[J]. Nat Protoc, 2008, 3(6): 1101-1108. DOI: 10.1038/nprot.2008.73. |
| [12] |
MCGEARY SE, LIN KS, SHI CY, et al. The biochemical basis of microRNA targeting efficacy[J]. Science, 2019, 366(6472): eaav1741. DOI: 10.1126/science.aav1741. |
| [13] |
ZOU Y, LI R, KUANG D, et al. Galangin inhibits cholangiocarcinoma cell growth and metastasis through downregulation of MicroRNA-21 expression[J]. Biomed Res Int, 2020, 2020: 5846938. DOI: 10.1155/2020/5846938. |
| [14] |
NARAYANAN S, CAI CY, ASSARAF YG, et al. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance[J]. Drug Resist Updat, 2020, 48: 100663. DOI: 10.1016/j.drup.2019.100663. |
| [15] |
MOLLAEI H, SAFARALIZADEH R, ROSTAMI Z. MicroRNA replacement therapy in cancer[J]. J Cell Physiol, 2019, 234(8): 12369-12384. DOI: 10.1002/jcp.28058. |
| [16] |
SALIMINEJAD K, KHORRAM KHORSHID HR, SOLEYMANI FARD S, et al. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods[J]. J Cell Physiol, 2019, 234(5): 5451-5465. DOI: 10.1002/jcp.27486. |
| [17] |
ROSSI M, ALTOMARE E, BOTTA C, et al. miR-21 antagonism abrogates Th17 tumor promoting functions in multiple myeloma[J]. Leukemia, 2021, 35(3): 823-834. DOI: 10.1038/s41375-020-0947-1. |
| [18] |
WANG H, TAN Z, HU H, et al. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1[J]. BMC Cancer, 2019, 19(1): 738. DOI: 10.1186/s12885-019-5951-3. |
| [19] |
WANG B, HUA P, ZHANG L, et al. LncRNA-IUR up-regulates PTEN by sponging miR-21 to regulate cancer cell proliferation and apoptosis in esophageal squamous cell carcinoma[J]. Esophagus, 2020, 17(3): 298-304. DOI: 10.1007/s10388-020-00724-x. |
| [20] |
TAN AC. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC)[J]. Thorac Cancer, 2020, 11(3): 511-518. DOI: 10.1111/1759-7714.13328. |
| [21] |
JANG DK, LEE YG, CHAN CHAE Y, et al. GDC-0980 (apitolisib) treatment with gemcitabine and/or cisplatin synergistically reduces cholangiocarcinoma cell growth by suppressing the PI3K/Akt/mTOR pathway[J]. Biochem Biophys Res Commun, 2020, 529(4): 1242-1248. DOI: 10.1016/j.bbrc.2020.06.011. |
| [22] |
ZHANG XM, LIU ZL, QIU B, et al. Downregulation of EVI1 expression inhibits cell proliferation and induces apoptosis in hilar cholangiocarcinoma via the PTEN/AKT signalling pathway[J]. J Cancer, 2020, 11(6): 1412-1423. DOI: 10.7150/jca.31903. |
| [23] |
LI G, ZHANG C, LIANG W, et al. Berberine regulates the Notch1/PTEN/PI3K/AKT/mTOR pathway and acts synergistically with 17-AAG and SAHA in SW480 colon cancer cells[J]. Pharm Biol, 2021, 59(1): 21-30. DOI: 10.1080/13880209.2020.1865407. |
| [24] |
LIU HY, ZHANG YY, ZHU BL, et al. miR-21 regulates the proliferation and apoptosis of ovarian cancer cells through PTEN/PI3K/AKT[J]. Eur Rev Med Pharmacol Sci, 2019, 23(10): 4149-4155. DOI: 10.26355/eurrev_201905_17917. |








 DownLoad:
DownLoad: