| [1] |
MCGLYNN KA, PETRICK JL, LONDON WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability[J]. Clin Liver Dis, 2015, 19(2): 223-238. DOI: 10.1016/j.cld.2015.01.001. |
| [2] |
SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. |
| [3] |
COURI T, PILLAI A. Goals and targets for personalized therapy for HCC[J]. Hepatol Int, 2019, 13(2): 125-137. DOI: 10.1007/s12072-018-9919-1. |
| [4] |
KANWAL F, SINGAL AG. Surveillance for hepatocellular carcinoma: current best practice and future direction[J]. Gastroenterology, 2019, 157(1): 54-64. DOI: 10.1053/j.gastro.2019.02.049. |
| [5] |
|
| [6] |
TANDON P, GARCIA-TSAO G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies[J]. Liver Int, 2009, 29(4): 502-510. DOI: 10.1111/j.1478-3231.2008.01957.x. |
| [7] |
EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389(10088): 2492-2502. DOI: 10.1016/S0140-6736(17)31046-2. |
| [8] |
YAU T, PARK JW, FINN RS, et al. CheckMate 459: A randomized, multi-center phase Ⅲ study of nivolumab (NⅣO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC)[J]. Ann Oncol, 2019, 30(5_suppl): 874-875. DOI: 10.1093/annonc/mdz394.029. |
| [9] |
FINN RS, RYOO BY, MERLE P, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC)[J]. J Clin Oncol, 2019, 37(15_suppl): 4004. DOI: 10.1200/JCO.2019.37.15_suppl.4004. |
| [10] |
SCHMITZ R, WRIGHT GW, HUANG DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma[J]. N Engl J Med, 2018, 378(15): 1396-1407. DOI: 10.1056/NEJMoa1801445. |
| [11] |
MUPPIDI JR, SCHMITZ R, GREEN JA, et al. Loss of signalling via Gα13 in germinal centre B-cell-derived lymphoma[J]. Nature, 2014, 516(7530): 254-258. DOI: 10.1038/nature13765. |
| [12] |
MUPPIDI JR, LU E, CYSTER JG. The G protein-coupled receptor P2RY8 and follicular dendritic cells promote germinal center confinement of B cells, whereas S1PR3 can contribute to their dissemination[J]. J Exp Med, 2015, 212(13): 2213-2222. DOI: 10.1084/jem.20151250. |
| [13] |
LU E, WOLFREYS FD, MUPPIDI JR, et al. S-Geranylgeranyl-L-glutathione is a ligand for human B cell-confinement receptor P2RY8[J]. Nature, 2019, 567(7747): 244-248. DOI: 10.1038/s41586-019-1003-z. |
| [14] |
PAGE EC, HEATLEY SL, EADIE LN, et al. HMGN1 plays a significant role in CRLF2 driven down syndrome leukemia and provides a potential therapeutic target in this high-risk cohort[J]. Oncogene, 2022, 41(6): 797-808. DOI: 10.1038/s41388-021-02126-4. |
| [15] |
AYPAR U, TAYLOR J, GARCIA JS, et al. P2RY8-CRLF2 fusion-positive acute myeloid leukemia with myelodysplasia-related changes: response to novel therapy[J]. JCO Precis Oncol, 2020, 4: 152-160. DOI: 10.1200/PO.19.00294. |
| [16] |
TOMCZAK K, CZERWIŃSKA P, WIZNEROWICZ M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge[J]. Contemp Oncol (Pozn), 2015, 19(1A): A68-A77. DOI: 10.5114/wo.2014.47136. |
| [17] |
RAVI R, NOONAN KA, PHAM V, et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy[J]. Nat Commun, 2018, 9(1): 741. DOI: 10.1038/s41467-017-02696-6. |
| [18] |
JIANG P, GU S, PAN D, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response[J]. Nat Med, 2018, 24(10): 1550-1558. DOI: 10.1038/s41591-018-0136-1. |
| [19] |
HE Y, GALLMAN AE, XIE C, et al. P2RY8 variants in lupus patients uncover a role for the receptor in immunological tolerance[J]. J Exp Med, 2022, 219(1): 7-33. DOI: 10.1084/jem.20211004. |
| [20] |
GREAVES SA, PETERSON JN, STRAUCH P, et al. Active PI3K abrogates central tolerance in high-avidity autoreactive B cells[J]. J Exp Med, 2019, 216(5): 1135-1153. DOI: 10.1084/jem.20181652. |
| [21] |
BOUMA G, BURNS SO, THRASHER AJ. Wiskott-Aldrich Syndrome: Immunodeficiency resulting from defective cell migration and impaired immunostimulatory activation[J]. Immunobiology, 2009, 214(9-10): 778-790. DOI: 10.1016/j.imbio.2009.06.009. |
| [22] |
BOURNAZOS S, CORTI D, VIRGIN HW, et al. Fc-optimized antibodies elicit CD8 immunity to viral respiratory infection[J]. Nature, 2020, 588(7838): 485-490. DOI: 10.1038/s41586-020-2838-z. |





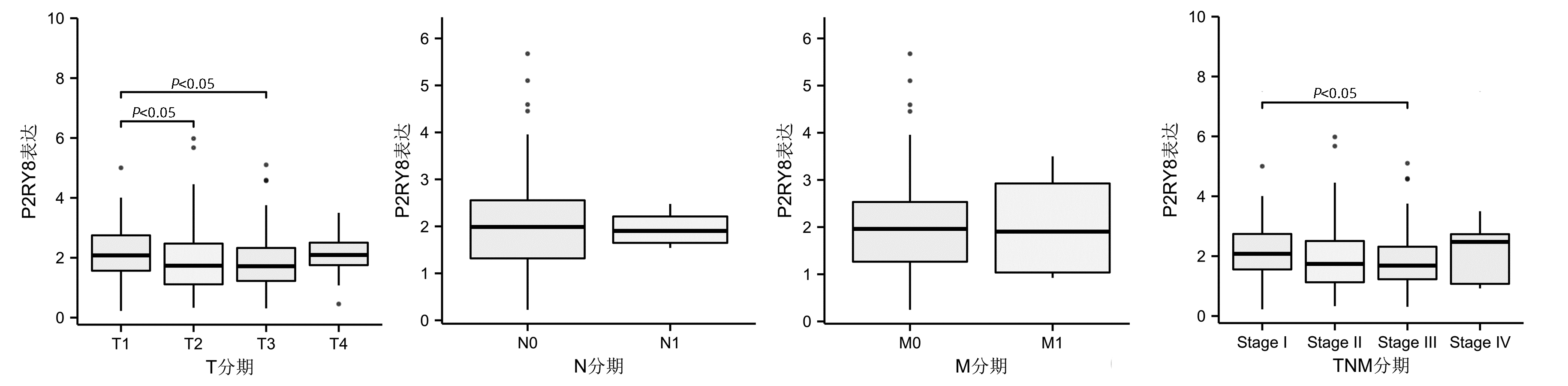




 DownLoad:
DownLoad:





