| [1] |
SIEGEL RL, MILLER KD, FUCHS HE, et al. Cancer statistics, 2021[J]. CA Cancer J Clin, 2021, 71(1): 7-33. DOI: 10.3322/caac.21654. |
| [2] |
NEOPTOLEMOS JP, KLEEFF J, MICHL P, et al. Therapeutic developments in pancreatic cancer: Current and future perspectives[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(6): 333-348. DOI: 10.1038/s41575-018-0005-x. |
| [3] |
RIBAS A, WOLCHOK JD. Cancer immunotherapy using checkpoint blockade[J]. Science, 2018, 359(6382): 1350-1355. DOI: 10.1126/science.aar4060. |
| [4] |
BEDNAR F, PASCA DI MAGLIANO M. Context-dependent immune responses explain pancreatic cancer immunoresistance[J]. Cancer Cell, 2020, 37(3): 261-263. DOI: 10.1016/j.ccell.2020.02.010. |
| [5] |
LIANG C, SHI S, MENG Q, et al. Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: Where we are and where we are going[J]. Exp Mol Med, 2017, 49(12): e406. DOI: 10.1038/emm.2017.255. |
| [6] |
POTHULA SP, XU Z, GOLDSTEIN D, et al. Key role of pancreatic stellate cells in pancreatic cancer[J]. Cancer Lett, 2016, 381(1): 194-200. DOI: 10.1016/j.canlet.2015.10.035. |
| [7] |
STEER A, CORDES N, JENDROSSEK V, et al. Impact of cancer-associated fibroblast on the radiation-response of solid xenograft tumors[J]. Front Mol Biosci, 2019, 6: 70. DOI: 10.3389/fmolb.2019.00070. |
| [8] |
OGIER C, COLOMBO PE, BOUSQUET C, et al. Targeting the NRG1/HER3 pathway in tumor cells and cancer-associated fibroblasts with an anti-neuregulin 1 antibody inhibits tumor growth in pre-clinical models of pancreatic cancer[J]. Cancer Lett, 2018, 432: 227-236. DOI: 10.1016/j.canlet.2018.06.023. |
| [9] |
HESLER RA, HUANG JJ, STARR MD, et al. TGF-β-induced stromal CYR61 promotes resistance to gemcitabine in pancreatic ductal adenocarcinoma through downregulation of the nucleoside transporters hENT1 and hCNT3[J]. Carcinogenesis, 2016, 37(11): 1041-1051. DOI: 10.1093/carcin/bgw093. |
| [10] |
LU JL, WANG LL, LIANG XL, et al. High-molecular-weight hyaluronan produced by activated pancreatic stellate cells promotes pancreatic cancer cell migration via paracrine signaling[J]. Biochem Biophys Res Commun, 2019, 515(3): 493-498. DOI: 10.1016/j.bbrc.2019.05.167. |
| [11] |
DUFORT CC, DELGIORNO KE, CARLSON MA, et al. Interstitial pressure in pancreatic ductal adenocarcinoma is dominated by a gel-fluid phase[J]. Biophys J, 2016, 110(9): 2106-2119. DOI: 10.1016/j.bpj.2016.03.040. |
| [12] |
GOEHRIG D, NIGRI J, SAMAIN R, et al. Stromal protein βig-h3 reprogrammes tumour microenvironment in pancreatic cancer[J]. Gut, 2019, 68(4): 693-707. DOI: 10.1136/gutjnl-2018-317570. |
| [13] |
HESSMANN E, PATZAK MS, KLEIN L, et al. Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer[J]. Gut, 2018, 67(3): 497-507. DOI: 10.1136/gutjnl-2016-311954. |
| [14] |
PUSCEDDU S, GHIDINI M, TORCHIO M, et al. Comparative effectiveness of gemcitabine plus nab-paclitaxel and FOLFIRINOX in the first-line setting of metastatic pancreatic cancer: A systematic review and meta-analysis[J]. Cancers (Basel), 2019, 11(4): 484. DOI: 10.3390/cancers11040484. |
| [15] |
AMRUTKAR M, GLADHAUG IP. Pancreatic cancer chemoresistance to gemcitabine[J]. Cancers (Basel), 2017, 9(11): 157. DOI: 10.3390/cancers9110157. |
| [16] |
AMRUTKAR M, VETHE NT, VERBEKE CS, et al. Differential gemcitabine sensitivity in primary human pancreatic cancer cells and paired stellate cells is driven by heterogenous drug uptake and processing[J]. Cancers (Basel), 2020, 12(12): 3628. DOI: 10.3390/cancers12123628. |
| [17] |
HESLER RA, HUANG JJ, STARR MD, et al. TGF-β-induced stromal CYR61 promotes resistance to gemcitabine in pancreatic ductal adenocarcinoma through downregulation of the nucleoside transporters hENT1 and hCNT3[J]. Carcinogenesis, 2016, 37(11): 1041-1051. DOI: 10.1093/carcin/bgw093. |
| [18] |
DALIN S, SULLIVAN MR, LAU AN, et al. Deoxycytidine release from pancreatic stellate cells promotes gemcitabine resistance[J]. Cancer Res, 2019, 79(22): 5723-5733. DOI: 10.1158/0008-5472.CAN-19-0960. |
| [19] |
LIU SL, CAO SG, LI Y, et al. Pancreatic stellate cells facilitate pancreatic cancer cell viability and invasion[J]. Oncol Lett, 2019, 17(2): 2057-2062. DOI: 10.3892/ol.2018.9816. |
| [20] |
AMRUTKAR M, AASRUM M, VERBEKE CS, et al. Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells[J]. BMC Cancer, 2019, 19(1): 596. DOI: 10.1186/s12885-019-5803-1. |
| [21] |
PERAN I, DAKSHANAMURTHY S, MCCOY MD, et al. Cadherin 11 promotes immunosuppression and extracellular matrix deposition to support growth of pancreatic tumors and resistance to gemcitabine in mice[J]. Gastroenterology, 2021, 160(4): 1359-1372. e13. DOI: 10.1053/j.gastro.2020.11.044. |
| [22] |
LEE J, YAKUBOV B, IVAN C, et al. Tissue transglutaminase activates cancer-associated fibroblasts and contributes to gemcitabine resistance in pancreatic cancer[J]. Neoplasia, 2016, 18(11): 689-698. DOI: 10.1016/j.neo.2016.09.003. |
| [23] |
WEI L, YE H, LI G, et al. Correction: Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer[J]. Cell Death Dis, 2021, 12(3): 232. DOI: 10.1038/s41419-021-03420-5. |
| [24] |
NEUMANN C, VON HÖRSCHELMANN E, REUTZEL-SELKE A, et al. Tumor-stromal cross-talk modulating the therapeutic response in pancreatic cancer[J]. Hepatobiliary Pancreat Dis Int, 2018, 17(5): 461-472. DOI: 10.1016/j.hbpd.2018.09.004. |
| [25] |
FELDMANN K, MAURER C, PESCHKE K, et al. Mesenchymal Plasticity regulated by prrx1 drives aggressive pancreatic cancer biology[J]. Gastroenterology, 2021, 160(1): 346-361. e24. DOI: 10.1053/j.gastro.2020.09.010. |
| [26] |
WEI L, LIN Q, LU Y, et al. Cancer-associated fibroblasts-mediated ATF4 expression promotes malignancy and gemcitabine resistance in pancreatic cancer via the TGF-β1/ SMAD2/3 pathway and ABCC1 transactivation[J]. Cell Death Dis, 2021, 12(4): 334. DOI: 10.1038/s41419-021-03574-2. |
| [27] |
IRELAND L, SANTOS A, AHMED MS, et al. Chemoresistance in pancreatic cancer is driven by stroma-derived insulin-like growth factors[J]. Cancer Res, 2016, 76(23): 6851-6863. DOI: 10.1158/0008-5472.CAN-16-1201. |
| [28] |
ZHANG D, LI L, JIANG H, et al. Tumor-Stroma IL1β-IRAK4 feedforward circuitry drives tumor fibrosis, chemoresistance, and poor prognosis in pancreatic cancer[J]. Cancer Res, 2018, 78(7): 1700-1712. DOI: 10.1158/0008-5472.CAN-17-1366. |
| [29] |
VENNIN C, MÉLÉNEC P, ROUET R, et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan[J]. Nat Commun, 2019, 10(1): 3637. DOI: 10.1038/s41467-019-10968-6. |
| [30] |
TOSTE PA, NGUYEN AH, KADERA BE, et al. Chemotherapy-induced inflammatory gene signature and protumorigenic phenotype in pancreatic CAFs via stress-associated MAPK[J]. Mol Cancer Res, 2016, 14(5): 437-447. DOI: 10.1158/1541-7786.MCR-15-0348. |
| [31] |
FANG Y, ZHOU W, RONG Y, et al. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer[J]. Exp Cell Res, 2019, 383(1): 111543. DOI: 10.1016/j.yexcr.2019.111543. |
| [32] |
CAO J, MA J, SUN L, et al. Targeting glypican-4 overcomes 5-FU resistance and attenuates stem cell-like properties via suppression of Wnt/β-catenin pathway in pancreatic cancer cells[J]. J Cell Biochem, 2018, 119(11): 9498-9512. DOI: 10.1002/jcb.27266. |
| [33] |
ZHOU T, LIU J, XIE Y, et al. ESE3/EHF, a promising target of rosiglitazone, suppresses pancreatic cancer stemness by downregulating CXCR4[J]. Gut, 2022, 71(2): 357-371. DOI: 10.1136/gutjnl-2020-321952. |
| [34] |
BEGUM A, MCMILLAN RH, CHANG YT, et al. Direct interactions with cancer-associated fibroblasts lead to enhanced pancreatic cancer stem cell function[J]. Pancreas, 2019, 48(3): 329-334. DOI: 10.1097/MPA.0000000000001249. |
| [35] |
CHAN TS, HSU CC, PAI VC, et al. Metronomic chemotherapy prevents therapy-induced stromal activation and induction of tumor-initiating cells[J]. J Exp Med, 2016, 213(13): 2967-2988. DOI: 10.1084/jem.20151665. |
| [36] |
KUEN J, DAROWSKI D, KLUGE T, et al. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model[J]. PLoS One, 2017, 12(7): e0182039. DOI: 10.1371/journal.pone.0182039. |
| [37] |
DAS S, SHAPIRO B, VUCIC EA, et al. Tumor cell-derived IL1β promotes desmoplasia and immune suppression in pancreatic cancer[J]. Cancer Res, 2020, 80(5): 1088-1101. DOI: 10.1158/0008-5472.CAN-19-2080. |
| [38] |
BLAIR AB, KIM VM, MUTH ST, et al. Dissecting the stromal signaling and regulation of myeloid cells and memory effector T cells in pancreatic cancer[J]. Clin Cancer Res, 2019, 25(17): 5351-5363. DOI: 10.1158/1078-0432.CCR-18-4192. |
| [39] |
FAN CS, CHEN LL, HSU TA, et al. Endothelial-mesenchymal transition harnesses HSP90α-secreting M2-macrophages to exacerbate pancreatic ductal adenocarcinoma[J]. J Hematol Oncol, 2019, 12(1): 138. DOI: 10.1186/s13045-019-0826-2. |
| [40] |
ZHANG A, QIAN Y, YE Z, et al. Cancer-associated fibroblasts promote M2 polarization of macrophages in pancreatic ductal adenocarcinoma[J]. Cancer Med, 2017, 6(2): 463-470. DOI: 10.1002/cam4.993. |
| [41] |
NAJAFI M, HASHEMI GORADEL N, FARHOOD B, et al. Macrophage polarity in cancer: A review[J]. J Cell Biochem, 2019, 120(3): 2756-2765. DOI: 10.1002/jcb.27646. |
| [42] |
van AUDENAERDE J, ROEYEN G, DARCY PK, et al. Natural killer cells and their therapeutic role in pancreatic cancer: A systematic review[J]. Pharmacol Ther, 2018, 189: 31-44. DOI: 10.1016/j.pharmthera.2018.04.003. |
| [43] |
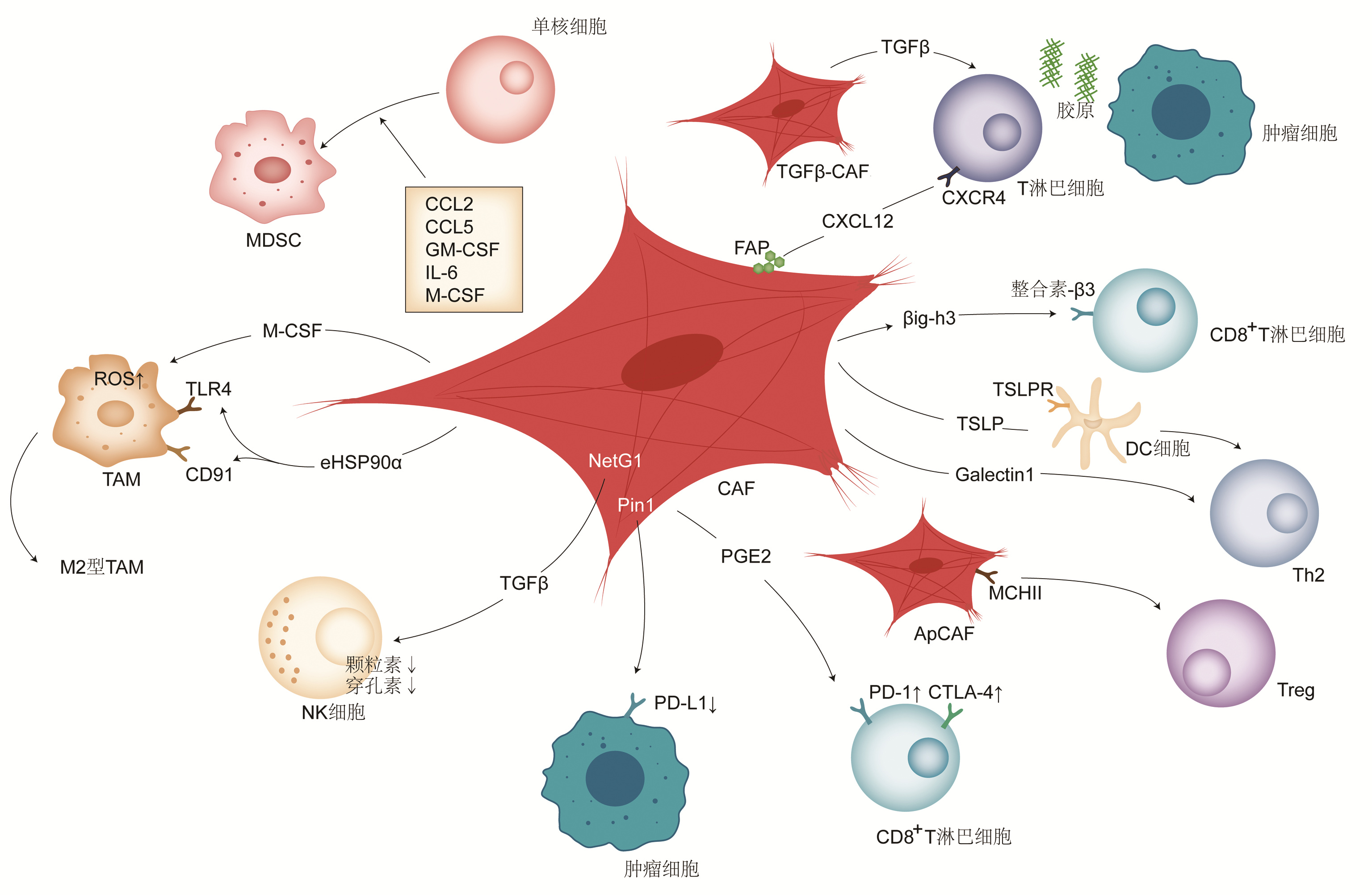
FRANCESCONE R, BARBOSA VENDRAMINI-COSTA D, FRANCO-BARRAZA J, et al. Netrin G1 promotes pancreatic tumorigenesis through cancer-associated fibroblast-driven nutritional support and immunosuppression[J]. Cancer Discov, 2021, 11(2): 446-479. DOI: 10.1158/2159-8290.CD-20-0775. |
| [44] |
WU Y, TIAN Z, WEI H. Developmental and functional control of natural killer cells by cytokines[J]. Front Immunol, 2017, 8: 930. DOI: 10.3389/fimmu.2017.00930. |
| [45] |
GARG B, GIRI B, MODI S, et al. NFκB in pancreatic stellate cells reduces infiltration of tumors by cytotoxic T cells and killing of cancer cells, via up-regulation of CXCL12[J]. Gastroenterology, 2018, 155(3): 880-891. e8. DOI: 10.1053/j.gastro.2018.05.051. |
| [46] |
DOMINGUEZ CX, MVLLER S, KEERTHIVASAN S, et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15 + myofibroblasts as a determinant of patient response to cancer immunotherapy[J]. Cancer Discov, 2020, 10(2): 232-253. DOI: 10.1158/2159-8290.CD-19-0644. |
| [47] |
WANG Y, GAO Z, DU X, et al. Co-inhibition of the TGF-β pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy[J]. Biomater Sci, 2020, 8(18): 5121-5132. DOI: 10.1039/d0bm00916d. |
| [48] |
ELYADA E, BOLISETTY M, LAISE P, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts[J]. Cancer Discov, 2019, 9(8): 1102-1123. DOI: 10.1158/2159-8290.CD-19-0094. |
| [49] |
TANG D, GAO J, WANG S, et al. Apoptosis and anergy of T cell induced by pancreatic stellate cells-derived galectin-1 in pancreatic cancer[J]. Tumour Biol, 2015, 36(7): 5617-5626. DOI: 10.1007/s13277-015-3233-5. |
| [50] |
OROZCO CA, MARTINEZ-BOSCH N, GUERRERO PE, et al. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk[J]. Proc Natl Acad Sci U S A, 2018, 115(16): e3769-e3778. DOI: 10.1073/pnas.1722434115. |
| [51] |
BRUNETTO E, de MONTE L, BALZANO G, et al. The IL-1/IL-1 3receptor axis and tumor cell released inflammasome adaptor ASC are key regulators of TSLP secretion by cancer associated fibroblasts in pancreatic cancer[J]. J Immunother Cancer, 2019, 7(1): 45. DOI: 10.1186/s40425-019-0521-4. |
| [52] |
GORCHS L, FERNÁNDEZ MORO C, BANKHEAD P, et al. Human pancreatic carcinoma-associated fibroblasts promote expression of co-inhibitory markers on CD4 + and CD8 + T-Cells[J]. Front Immunol, 2019, 10: 847. DOI: 10.3389/fimmu.2019.00847. |
| [53] |
KOIKAWA K, KIBE S, SUIZU F, et al. Targeting Pin1 renders pancreatic cancer eradicable by synergizing with immunochemotherapy[J]. Cell, 2021, 184(18): 4753-4771. e27. DOI: 10.1016/j.cell.2021.07.020. |
| [54] |
WEN Z, LIU Q, WU J, et al. Fibroblast activation protein α-positive pancreatic stellate cells promote the migration and invasion of pancreatic cancer by CXCL1-mediated Akt phosphorylation[J]. Ann Transl Med, 2019, 7(20): 532. DOI: 10.21037/atm.2019.09.164. |
| [55] |
FEIG C, JONES JO, KRAMAN M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer[J]. Proc Natl Acad Sci U S A, 2013, 110(50): 20212-20217. DOI: 10.1073/pnas.1320318110. |
| [56] |
ZHANG Y, ERTL HC. Depletion of FAP+ cells reduces immunosuppressive cells and improves metabolism and functions CD8 +T cells within tumors[J]. Oncotarget, 2016, 7(17): 23282-23299. DOI: 10.18632/oncotarget.7818. |
| [57] |
WANG Y, LIANG Y, XU H, et al. Single-cell analysis of pancreatic ductal adenocarcinoma identifies a novel fibroblast subtype associated with poor prognosis but better immunotherapy response[J]. Cell Discov, 2021, 7(1): 36. DOI: 10.1038/s41421-021-00271-4. |








 DownLoad:
DownLoad: