| [1] |
EL-SERAG HB. Epidemiology of viral hepatitis and hepatocellular carcinoma[J]. Gastroenterology, 2012, 142(6): 1264-1273. e1. DOI: 10.1053/j.gastro.2011.12.061. |
| [2] |
MILOSEVIC I, VUJOVIC A, BARAC A, et al. Gut-liver axis, gut microbiota, and its modulation in the management of liver diseases: A review of the literature[J]. Int J Mol Sci, 2019, 20(2): 395. DOI: 10.3390/ijms20020395. |
| [3] |
LEY RE, PETERSON DA, GORDON JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine[J]. Cell, 2006, 124(4): 837-848. DOI: 10.1016/j.cell.2006.02.017. |
| [4] |
UBEDA C, DJUKOVIC A, ISAAC S. Roles of the intestinal microbiota in pathogen protection[J]. Clin Transl Immunology, 2017, 6(2): e128. DOI: 10.1038/cti.2017.2. |
| [5] |
|
| [6] |
LIU J, LIANG W, JING W, et al. Countdown to 2030: eliminating hepatitis B disease, China[J]. Bull World Health Organ, 2019, 97(3): 230-238. DOI: 10.2471/BLT.18.219469. |
| [7] |
WANG J, WANG Y, ZHANG X, et al. Gut microbial dysbiosis is associated with altered hepatic functions and serum metabolites in chronic hepatitis B patients[J]. Front Microbiol, 2017, 8: 2222. DOI: 10.3389/fmicb.2017.02222. |
| [8] |
ZENG Y, CHEN S, FU Y, et al. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma[J]. J Viral Hepat, 2020, 27(2): 143-155. DOI: 10.1111/jvh.13216. |
| [9] |
ZHU Q, XIA P, ZHOU X, et al. Hepatitis B virus infection alters gut microbiota composition in mice[J]. Front Cell Infect Microbiol, 2019, 9: 377. DOI: 10.3389/fcimb.2019.00377. |
| [10] |
CHOU HH, CHIEN WH, WU LL, et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota[J]. Proc Natl Acad Sci U S A, 2015, 112(7): 2175-2180. DOI: 10.1073/pnas.1424775112. |
| [11] |
CHEN Z, XIE Y, ZHOU F, et al. Featured gut microbiomes associated with the progression of chronic hepatitis B disease[J]. Front Microbiol, 2020, 11: 383. DOI: 10.3389/fmicb.2020.00383. |
| [12] |
GEHRING AJ, PROTZER U. Targeting innate and adaptive immune responses to cure chronic HBV infection[J]. Gastroenterology, 2019, 156(2): 325-337. DOI: 10.1053/j.gastro.2018.10.032. |
| [13] |
GUO W, ZHOU X, LI X, et al. Depletion of gut microbiota impairs gut barrier function and antiviral immune defense in the liver[J]. Front Immunol, 2021, 12: 636803. DOI: 10.3389/fimmu.2021.636803. |
| [14] |
URDANETA V, CASADESÚS J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts[J]. Front Med (Lausanne), 2017, 4: 163. DOI: 10.3389/fmed.2017.00163. |
| [15] |
SAYIN SI, WAHLSTRÖM A, FELIN J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist[J]. Cell Metab, 2013, 17(2): 225-235. DOI: 10.1016/j.cmet.2013.01.003. |
| [16] |
WANG X, CHEN L, WANG H, et al. Modulation of bile acid profile by gut microbiota in chronic hepatitis B[J]. J Cell Mol Med, 2020, 24(4): 2573-2581. DOI: 10.1111/jcmm.14951. |
| [17] |
KOH A, de VADDER F, KOVATCHEVA-DATCHARY P, et al. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites[J]. Cell, 2016, 165(6): 1332-1345. DOI: 10.1016/j.cell.2016.05.041. |
| [18] |
BAJAJ JS, REDDY KR, O'LEARY JG, et al. Serum levels of metabolites produced by intestinal microbes and lipid moieties independently associated with acute-on-chronic liver failure and death in patients with cirrhosis[J]. Gastroenterology, 2020, 159(5): 1715-1730. e12. DOI: 10.1053/j.gastro.2020.07.019. |
| [19] |
|
| [20] |
INOUE T, NAKAYAMA J, MORIYA K, et al. Gut dysbiosis associated with hepatitis C virus infection[J]. Clin Infect Dis, 2018, 67(6): 869-877. DOI: 10.1093/cid/ciy205. |
| [21] |
WELLHÖNER F, DÖSCHER N, WOELFL F, et al. Eradication of chronic HCV infection: Improvement of dysbiosis only in patients without liver cirrhosis[J]. Hepatology, 2021, 74(1): 72-82. DOI: 10.1002/hep.31700. |
| [22] |
PÉREZ-MATUTE P, ÍÑIGUEZ M, VILLANUEVA-MILLÁN MJ, et al. Short-term effects of direct-acting antiviral agents on inflammation and gut microbiota in hepatitis C-infected patients[J]. Eur J Intern Med, 2019, 67: 47-58. DOI: 10.1016/j.ejim.2019.06.005. |
| [23] |
PONZIANI FR, PUTIGNANI L, PARONI STERBINI F, et al. Influence of hepatitis C virus eradication with direct-acting antivirals on the gut microbiota in patients with cirrhosis[J]. Aliment Pharmacol Ther, 2018, 48(11-12): 1301-1311. DOI: 10.1111/apt.15004. |
| [24] |
SULTAN S, EL-MOWAFY M, ELGAML A, et al. Alterations of the treatment-naive gut microbiome in newly diagnosed hepatitis C virus infection[J]. ACS Infect Dis, 2021, 7(5): 1059-1068. DOI: 10.1021/acsinfecdis.0c00432. |
| [25] |
SARIN SK, CHOUDHURY A, SHARMA MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update[J]. Hepatol Int, 2019, 13(4): 353-390. DOI: 10.1007/s12072-019-09946-3. |
| [26] |
LI L, WU Z, MA W, et al. Changes in intestinal microflora in patients with chronic severe hepatitis[J]. Chin Med J (Engl), 2001, 114(8): 869-872.
|
| [27] |
YAO X, YU H, FAN G, et al. Impact of the gut microbiome on the progression of hepatitis B virus related acute-on-chronic liver failure[J]. Front Cell Infect Microbiol, 2021, 11: 573923. DOI: 10.3389/fcimb.2021.573923. |
| [28] |
WANG K, ZHANG Z, MO ZS, et al. Gut microbiota as prognosis markers for patients with HBV-related acute-on-chronic liver failure[J]. Gut Microbes, 2021, 13(1): 1-15. DOI: 10.1080/19490976.2021.1921925. |
| [29] |
DUPONT HL. Review article: the antimicrobial effects of rifaximin on the gut microbiota[J]. Aliment Pharmacol Ther, 2016, 43 (Suppl 1): 3-10. DOI: 10.1111/apt.13434. |
| [30] |
SHARMA BC, SHARMA P, LUNIA MK, et al. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy[J]. Am J Gastroenterol, 2013, 108(9): 1458-1463. DOI: 10.1038/ajg.2013.219. |
| [31] |
KANG SH, LEE YB, LEE JH, et al. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy[J]. Aliment Pharmacol Ther, 2017, 46(9): 845-855. DOI: 10.1111/apt.14275. |
| [32] |
GOEL A, RAHIM U, NGUYEN LH, et al. Systematic review with meta-analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitis[J]. Aliment Pharmacol Ther, 2017, 46(11-12): 1029-1036. DOI: 10.1111/apt.14361. |
| [33] |
BAJAJ JS. Review article: potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis[J]. Aliment Pharmacol Ther, 2016, 43 (Suppl 1): 11-26. DOI: 10.1111/apt.13435. |
| [34] |
BAJAJ JS, HEUMAN DM, SANYAL AJ, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy[J]. PLoS One, 2013, 8(4): e60042. DOI: 10.1371/journal.pone.0060042. |
| [35] |
CHAUHAN A, KUMAR R, SHARMA S, et al. Fecal microbiota transplantation in hepatitis B e antigen-positive chronic hepatitis B patients: A pilot study[J]. Dig Dis Sci, 2021, 66(3): 873-880. DOI: 10.1007/s10620-020-06246-x. |
| [36] |
REN YD, YE ZS, YANG LZ, et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy[J]. Hepatology, 2017, 65(5): 1765-1768. DOI: 10.1002/hep.29008. |
| [37] |
BAJAJ JS, KASSAM Z, FAGAN A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial[J]. Hepatology, 2017, 66(6): 1727-1738. DOI: 10.1002/hep.29306. |
| [38] |
BAJAJ JS, SALZMAN N, ACHARYA C, et al. Microbial functional change is linked with clinical outcomes after capsular fecal transplant in cirrhosis[J]. JCI Insight, 2019, 4(24) : e133410. DOI: 10.1172/jci.insight.133410. |
| [39] |
DEFILIPP Z, BLOOM PP, TORRES SOTO M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant[J]. N Engl J Med, 2019, 381(21): 2043-2050. DOI: 10.1056/NEJMoa1910437. |
| [40] |
Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of hepatic encephalopathy in cirrhosis[J]. J Clin Hepatol, 2018, 34(10): 2076-2089. DOI: 10.3969/j.issn.1001-5256.2018.10.007. |
| [41] |
ZHENG N, YU LL, CAO Z. Effect of treatment with viable bifidobacterium quadruple on liver function, cytokines and intestinal flora in patients with liver cirrhosis[J]. Med & Pharm J Chin PLA, 2020, 32(8): 36-39. DOI: 10.3969/j.issn.2095-140X.2020.08.009. |
| [42] |
DHIMAN RK, RANA B, AGRAWAL S, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial[J]. Gastroenterology, 2014, 147(6): 1327-1337. e3. DOI: 10.1053/j.gastro.2014.08.031. |
| [43] |
XIA X, CHEN J, XIA J, et al. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis[J]. J Int Med Res, 2018, 46(9): 3596-3604. DOI: 10.1177/0300060518776064. |
| [44] |
Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. |
| [45] |
YAN H, PENG B, LIU Y, et al. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide[J]. J Virol, 2014, 88(6): 3273-3284. DOI: 10.1128/JVI.03478-13. |
| [46] |
MOUZANNAR K, FUSIL F, LACOMBE B, et al. Farnesoid X receptor-α is a proviral host factor for hepatitis B virus that is inhibited by ligands in vitro and in vivo[J]. FASEB J, 2019, 33(2): 2472-2483. DOI: 10.1096/fj.201801181R. |
| [47] |
ZHANG DY, ZHU L, LIU HN, et al. The protective effect and mechanism of the FXR agonist obeticholic acid via targeting gut microbiota in non-alcoholic fatty liver disease[J]. Drug Des Devel Ther, 2019, 13: 2249-2270. DOI: 10.2147/DDDT.S207277. |
| [48] |
FRIEDMAN ES, LI Y, SHEN TD, et al. FXR-dependent modulation of the human small intestinal microbiome by the bile acid derivative obeticholic acid[J]. Gastroenterology, 2018, 155(6): 1741-1752. e5. DOI: 10.1053/j.gastro.2018.08.022. |
| [49] |
MARKHAM A, KEAM SJ. Obeticholic acid: First global approval[J]. Drugs, 2016, 76(12): 1221-1226. DOI: 10.1007/s40265-016-0616-x. |



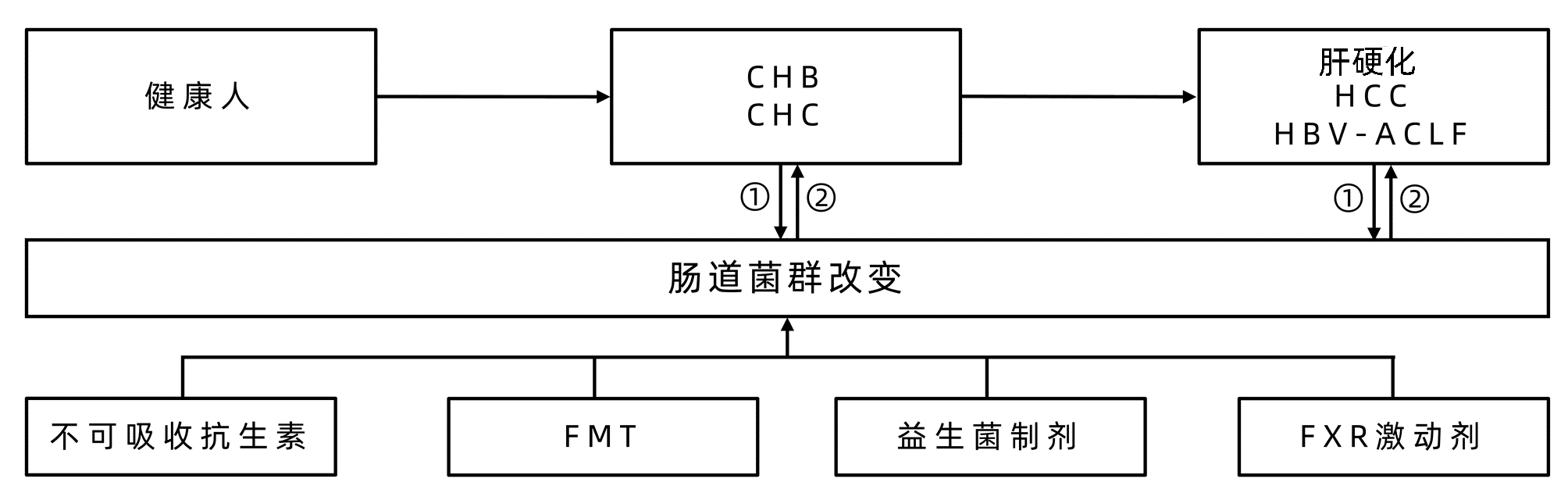




 DownLoad:
DownLoad: