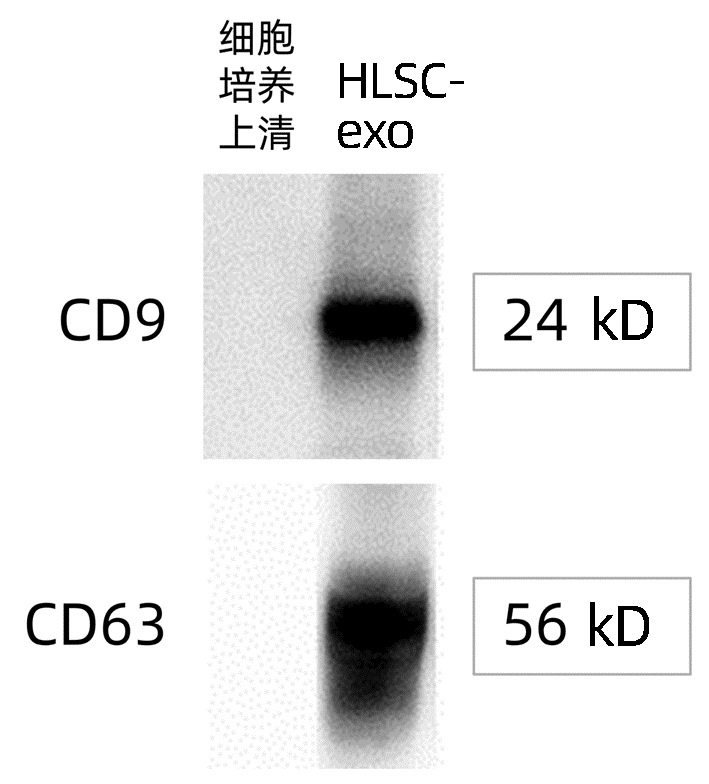
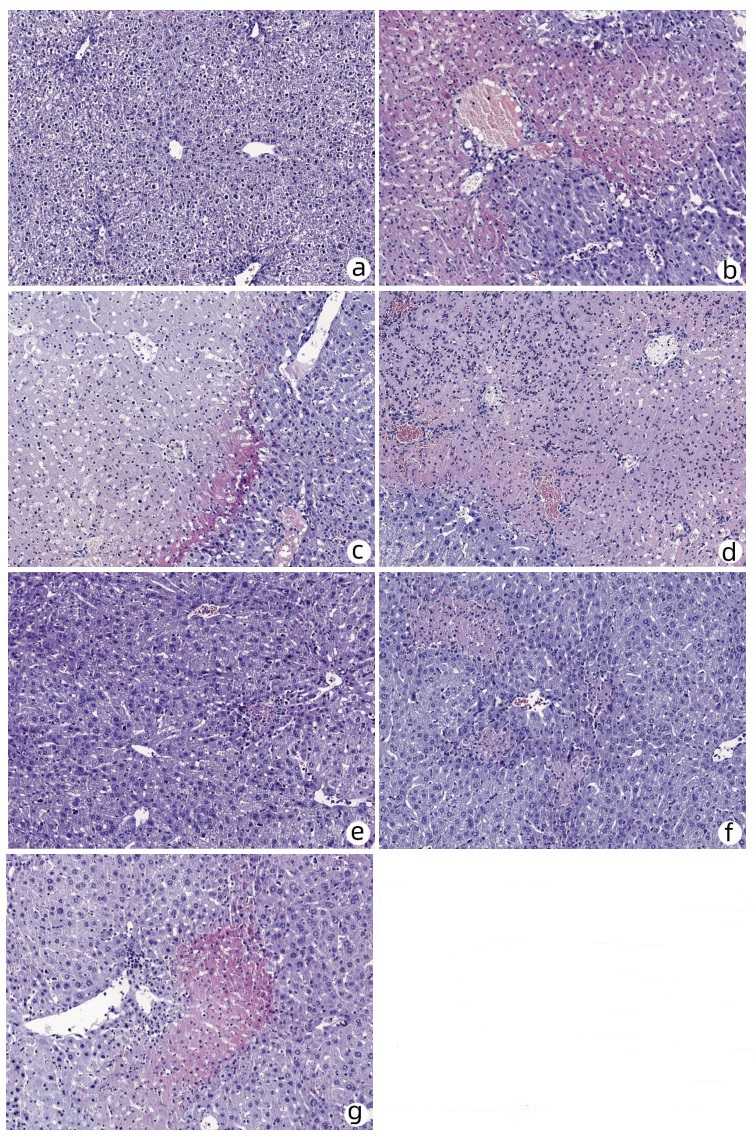
| [1] |
ESCORSELL À, CASTELLOTE J, SÁNCHEZ-DELGADO J, et al. Management of acute liver failure. Clinical guideline from the Catalan Society of Digestology[J]. Gastroenterol Hepatol, 2019, 42(1): 51-64. DOI: 10.1016/j.gastrohep.2018.07.013. |
| [2] |
YE T, WANG T, YANG X, et al. Comparison of concanavalin a-induced murine autoimmune hepatitis models[J]. Cell Physiol Biochem, 2018, 46(3): 1241-1251. DOI: 10.1159/000489074. |
| [3] |
JIANG W, TAN Y, CAI M, et al. Human umbilical cord MSC-derived exosomes suppress the development of CCl 4-induced liver injury through antioxidant effect[J]. Stem Cells Int, 2018, 2018: 6079642. DOI: 10.1155/2018/6079642. |
| [4] |
BI Y, LI J, YANG Y, et al. Human liver stem cells attenuate concanavalin A-induced acute liver injury by modulating myeloid-derived suppressor cells and CD4 + T cells in mice[J]. Stem Cell Res Ther, 2019, 10(1): 22. DOI: 10.1186/s13287-018-1128-2. |
| [5] |
FAN Z, ZHANG K, HONG F, et al. The mechanisms of intraperitoneal transplantation of human liver-derived stem cells against concanavalin A-induced acute liver injury in mice[J]. Chin J Lab Diag, 2018, 22(6): 1070-1073. DOI: 10.3969/j.issn.1007-4287.2018.06.043. |
| [6] |
HU C, ZHAO L, ZHANG L, et al. Mesenchymal stem cell-based cell-free strategies: Safe and effective treatments for liver injury[J]. Stem Cell Res Ther, 2020, 11(1): 377. DOI: 10.1186/s13287-020-01895-1. |
| [7] |
LOU G, CHEN Z, ZHENG M, et al. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases[J]. Exp Mol Med, 2017, 49(6): e346. DOI: 10.1038/emm.2017.63. |
| [8] |
SHIREJINI SZ, INCI F. The Yin and Yang of exosome isolation methods: conventional practice, microfluidics, and commercial kits[J]. Biotechnol Adv, 2022, 54: 107814. DOI: 10.1016/j.biotechadv.2021.107814. |
| [9] |
WANG L, YANG RN. Study on role and clinical significance of IL-9, IL-10 in immune liver damage[J]. Lab Med Clin, 2017, 14(20): 3011-3014. DOI: 10.3969/j.issn.1672-9455.2017.20.014. |
| [10] |
GAO YD, TIAN Y, CHEN Y, et al. Effect of magnesium isoglycyrrhizinate on concanavalin A-induced acute liver failure in mice[J]. J Clin Hepatol, 2020, 36(7): 1571-1576. DOI: 10.3969/j.issn.1001-5256.2020.07.024. |








 DownLoad:
DownLoad: