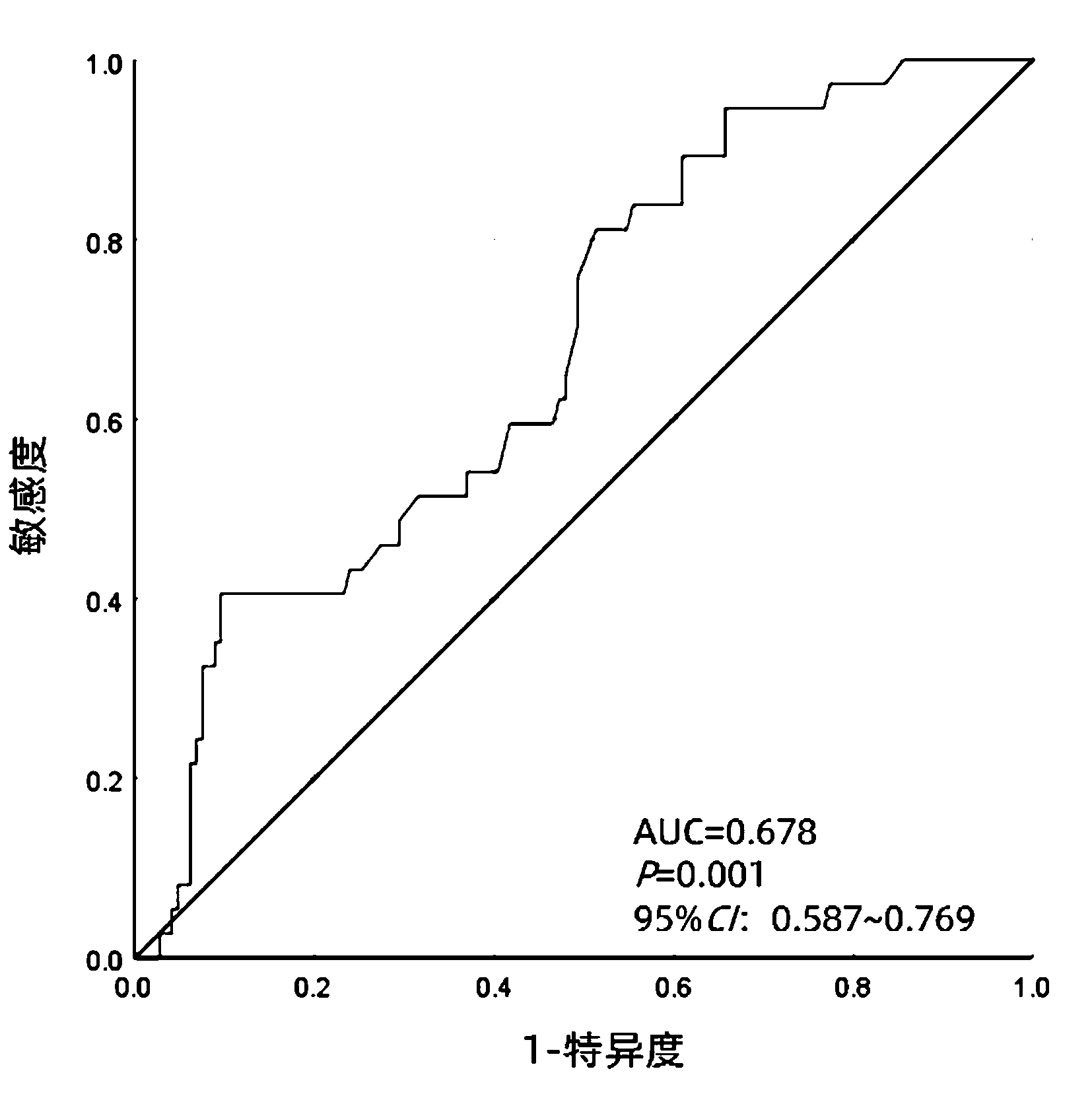
| [1] |
TAN M, BHADORIA AS, CUI F, et al. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: A systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2021, 6(2): 106-119. DOI: 10.1016/S2468-1253(20)30307-1. |
| [2] |
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. |
| [3] |
Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. |
| [4] |
KUMAR M, SARIN SK, HISSAR S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT[J]. Gastroenterology, 2008, 134(5): 1376-1384. DOI: 10.1053/j.gastro.2008.02.075. |
| [5] |
IANNACONE M, GUIDOTTI LG. Immunobiology and pathogenesis of hepatitis B virus infection[J]. Nat Rev Immunol, 2022, 22(1): 19-32. DOI: 10.1038/s41577-021-00549-4. |
| [6] |
CAI Y, PAN L, LIN B, et al. Relationship between Treg/Th17 balance and changes in HBeAg among HBeAg-positive chronic hepatitis B patients receiving entecavir therapy[J]. Int J Virol, 2020, 27(5): 407-411. DOI: 10.3760/cma.j.issn.1673-4092.2020.05.013. |
| [7] |
VLACHOGIANNAKOS J, PAPATHEODORIDIS GV. Hepatitis B: Who and when to treat?[J]. Liver Int, 2018, 38 Suppl 1: 71-78. DOI: 10.1111/liv.13631. |
| [8] |
GAO P, LUO Y, CHEN L, et al. The effect of hepatitis B virus on T lymphocyte and its subsets in chronic hepatitis B patients in different ALT stages: A new concept ALT in HBV infection[J]. Int Immunopharmacol, 2021, 101(Pt A): 108182. DOI: 10.1016/j.intimp.2021.108182. |
| [9] |
SEONG G, SINN DH, KANG W, et al. Age and fibrosis index for the prediction of hepatocellular carcinoma risk in patients with high hepatitis B virus DNA but normal alanine aminotransferase[J]. Eur J Gastroenterol Hepatol, 2022, 34(1): 69-75. DOI: 10.1097/MEG.0000000000001915. |
| [10] |
KIM GA, LIM YS, HAN S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B[J]. Gut, 2018, 67(5): 945-952. DOI: 10.1136/gutjnl-2017-314904. |
| [11] |
LI MH, XIE Y. Antiviral therapy is not recommended for patients in the immune-tolerant phase of hepatitis B virus infection[J]. J Clin Hepatol, 2021, 37(4): 783-784. DOI: 10.3969/j.issn.1001-5256.2021.04.011. |
| [12] |
LEE HA, LEE HW, KIM IH, et al. Extremely low risk of hepatocellular carcinoma development in patients with chronic hepatitis B in immune-tolerant phase[J]. Aliment Pharmacol Ther, 2020, 52(1): 196-204. DOI: 10.1111/apt.15741. |
| [13] |
JIANG XY, HUANG B, HUANG DP, et al. Long-term follow-up of cumulative incidence of hepatocellular carcinoma in hepatitis B virus patients without antiviral therapy[J]. World J Gastroenterol, 2021, 27(11): 1101-1116. DOI: 10.3748/wjg.v27.i11.1101. |
| [14] |
CHI Z, ZHAO W, LI JW, et al. Combination of quantitative hepatitis B core antibody (qHBcAb) and aspartate aminotransferase (AST) can accurately diagnose immune tolerance of chronic hepatitis B virus infection based on liver biopsy[J]. Clin Res Hepatol Gastroenterol, 2021, 45(6): 101563. DOI: 10.1016/j.clinre.2020.10.008. |
| [15] |
TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. |
| [16] |
LU J. A study on the influence of ALT, AST and ALP on the occurrence and development of viral hepatitis[J]. Labeled Immunoassays Clin Med, 2020, 27(1): 67-69, 95. DOI: 10.11748/bjmy.issn.1006-1703.2020.01.015. |
| [17] |
VAILLANT A. HBsAg, subviral particles, and their clearance in establishing a functional cure of chronic hepatitis B virus infection[J]. ACS Infect Dis, 2021, 7(6): 1351-1368. DOI: 10.1021/acsinfecdis.0c00638. |
| [18] |
LI MH, CHEN QQ, ZHANG L, et al. Association of cytokines with hepatitis B virus and its antigen[J]. J Med Virol, 2020. DOI: 10.1002/jmv.26301. |
| [19] |
LIN YJ, SUN FF, ZENG Z, et al. Combination and intermittent therapy based on pegylated interferon Alfa-2a for chronic hepatitis B with Nucleoside (Nucleotide) analog-experienced resulting in hepatitis B surface antigen clearance: A case report[J]. Viral Immunol, 2022, 35(1): 71-75. DOI: 10.1089/vim.2021.0112. |
| [20] |
LI MH, ZHANG L, QU XJ, et al. The predictive value of baseline HBsAg level and early response for HBsAg loss in patients with HBeAg-positive Chronic Hepatitis B during pegylated interferon alpha-2a treatment[J]. Biomed Environ Sci, 2017, 30(3): 177-184. DOI: 10.3967/bes2017.025. |
| [21] |
PAN CQ, LI MH, YI W, et al. Outcome of Chinese patients with hepatitis B at 96 weeks after functional cure with IFN versus combination regimens[J]. Liver Int, 2021, 41(7): 1498-1508. DOI: 10.1111/liv.14801. |
| [22] |
LI M, ZHANG L, LU Y, et al. Early serum HBsAg kinetics as predictor of HBsAg loss in patients with HBeAg-Negative chronic hepatitis B after treatment with pegylated interferonα-2a[J]. Virol Sin, 2021, 36(2): 311-320. DOI: 10.1007/s12250-020-00290-7. |
| [23] |
CHAN HL, WONG VW, WONG GL, et al. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B[J]. Hepatology, 2010, 52(4): 1232-1241. DOI: 10.1002/hep.23803. |
| [24] |
LIU J, WANG J, YAN X, et al. Presence of liver inflammation in Asian patients with chronic hepatitis B with normal ALT and detectable HBV DNA in absence of liver fibrosis[J]. Hepatol Commun, 2021. DOI: 10.1002/hep4.1859. |



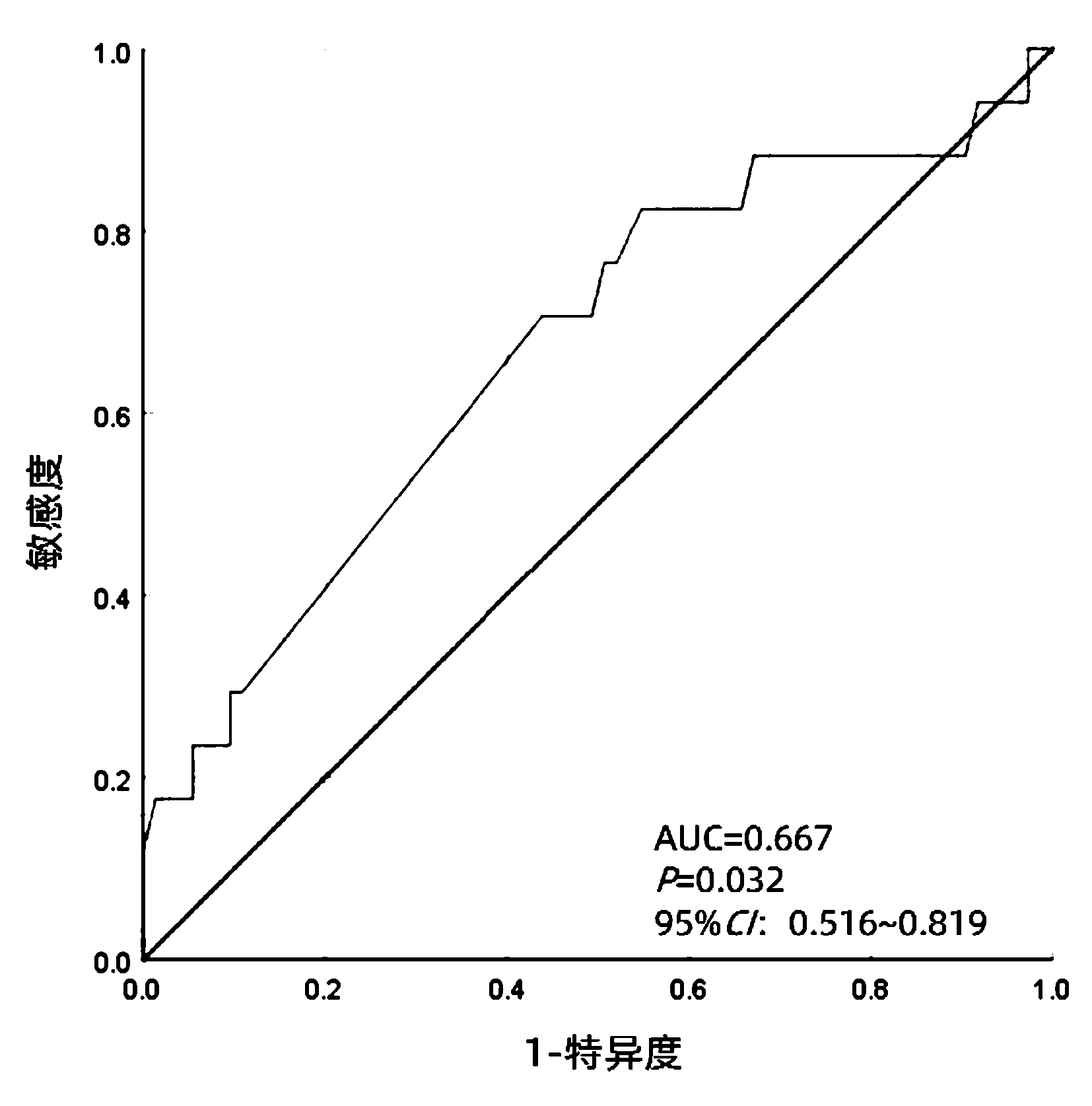




 DownLoad:
DownLoad: