| [1] |
SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. |
| [2] |
ZHOU M, WANG H, ZENG X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2019, 394(10204): 1145-1158. DOI: 10.1016/S0140-6736(19)30427-1. |
| [3] |
|
| [4] |
|
| [5] |
Chinese Society of Clinical Oncology Guidelines Working Committee. Chinese Society of Clinical Oncology(CSCO) guidelines for the diagnosis and treatment of primary liver cancer 2020[M]. Beijing: People's Health Publishing House, 2020.
中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)原发性肝癌诊疗指南2020[M]. 北京: 人民卫生出版社, 2020.
|
| [6] |
The Chinese Chapter of the International Hepato-Pancreato-Biliary Association; Group of Liver Surgery, Surgical Society of Chinese Medical Association; Expert Committee on Liver Cancer, Chinese Society of Clinical Oncology. Chinese multidisciplinary expert consensus on combined immunotherapy based on immune checkpoint inhibitors for hepatocellular carcinoma (2021 version) [J]. Chin J Dig Surg, 2021, 20(7): 740-753. DOI: 10.3760/cma.j.cn115610-20210603-00260. |
| [7] |
LLOVET JM, KELLEY RK, VILLANUEVA A, et al. Hepatocellular carcinoma[J]. Nat Rev Dis Primers, 2021, 7(1): 6. DOI: 10.1038/s41572-020-00240-3. |
| [8] |
HOSHIDA Y, VILLANUEVA A, KOBAYASHI M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma[J]. N Engl J Med, 2008, 359(19): 1995-2004. DOI: 10.1056/NEJMoa0804525. |
| [9] |
CHENG AL, KANG YK, CHEN Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase Ⅲ randomised, double-blind, placebo-controlled trial[J]. Lancet Oncol, 2009, 10(1): 25-34. DOI: 10.1016/S1470-2045(08)70285-7. |
| [10] |
KUDO M, FINN RS, QIN S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391(10126): 1163-1173. DOI: 10.1016/S0140-6736(18)30207-1. |
| [11] |
QIN S, BI F, GU S, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: A randomized, open-label, parallel-controlled phase Ⅱ-Ⅲ trial[J]. J Clin Oncol, 2021, 39(27): 3002-3011. DOI: 10.1200/JCO.21.00163. |
| [12] |
BRUIX J, QIN S, MERLE P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2017, 389(10064): 56-66. DOI: 10.1016/S0140-6736(16)32453-9. |
| [13] |
QIN S, LI Q, GU S, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial[J]. Lancet Gastroenterol Hepatol, 2021, 6(7): 559-568. DOI: 10.1016/S2468-1253(21)00109-6. |
| [14] |
ABOU-ALFA GK, MEYER T, CHENG AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma[J]. N Engl J Med, 2018, 379(1): 54-63. DOI: 10.1056/NEJMoa1717002. |
| [15] |
FINN RS, QIN S, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20): 1894-1905. DOI: 10.1056/NEJMoa1915745. |
| [16] |
ZHU AX, KANG YK, YEN CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Oncol, 2019, 20(2): 282-296. DOI: 10.1016/S1470-2045(18)30937-9. |
| [17] |
EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389(10088): 2492-2502. DOI: 10.1016/S0140-6736(17)31046-2. |
| [18] |
ZHU AX, FINN RS, EDELINE J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial[J]. Lancet Oncol, 2018, 19(7): 940-952. DOI: 10.1016/S1470-2045(18)30351-6. |
| [19] |
QIN S, REN Z, MENG Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial[J]. Lancet Oncol, 2020, 21(4): 571-580. DOI: 10.1016/S1470-2045(20)30011-5. |
| [20] |
REN Z, XU J, BAI Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study[J]. Lancet Oncol, 2021, 22(7): 977-990. DOI: 10.1016/S1470-2045(21)00252-7. |
| [21] |
DUCREUX M, ABOU-ALFA GK, REN Z, et al. Results from a global Phase 2 study of tislelizumab, an investigational PD-1 antibody, in patients with previously treated advanced hepatocellular carcinoma[J]. Ann Oncol, 2021, 32(3s): s217.
|
| [22] |
YAU T, KANG YK, KIM TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial[J]. JAMA Oncol, 2020, 6(11): e204564. DOI: 10.1001/jamaoncol.2020.4564. |
| [23] |
FINN RS, QIN S, IKEDA M, et al. IMbrave150: Updated overall survival data from a global, randomized, open-label Phase Ⅲ study of atezolizumab+bevacizumab vs sorafenib in patients with unresectable hepatocellular carcinoma[R]. J Clin Oncol, 2021, 39(3s): abstract 267.
|
| [24] |
FINN RS, IKEDA M, ZHU AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma[J]. J Clin Oncol, 2020, 38(26): 2960-2970. DOI: 10.1200/JCO.20.00808. |
| [25] |
KELLEY RK, YAU T, CHENG AL, et al. Cabozantinib plus atezolizumab versus sorafenib as first-line systemic treatment for advanced hepatocellular carcinoma: Results from the randomized phase 3 COSMIC-312 trial[R]. Esmo Asia Virtual Plenary, 2021, Abstract VP10-2021.
|
| [26] |
LLOVET JM, VILLANUEVA A, MARRERO JA, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference[J]. Hepatology, 2021, 73(Suppl 1): 158-191. DOI: 10.1002/hep.31327. |
| [27] |
LLOVET JM, LENCIONI R. mRECIST for HCC: Performance and novel refinements[J]. J Hepatol, 2020, 72(2): 288-306. DOI: 10.1016/j.jhep.2019.09.026. |
| [28] |
SUN L, MU L, ZHOU J, et al. Imaging features of gadoxetic acid-enhanced MR imaging for evaluation of tumor-infiltrating CD8 cells and PD-L1 expression in hepatocellular carcinoma[J]. Cancer Immunol Immunother, 2022, 71(1): 25-38. DOI: 10.1007/s00262-021-02957-w. |
| [29] |
KUDO M, UESHIMA K, NAKAHIRA S, et al. Adjuvant nivolumab for hepatocellular carcinoma (HCC) after surgical resection (SR) or radiofrequency ablation (RFA) (NIVOLVE): A phase 2 prospective multicenter single-arm trial and exploratory biomarker analysis[R]. 2021 ASCO, Abstract 4093.
|
| [30] |
LIAO H, ZHANG Z, CHEN J, et al. Preoperative radiomic approach to evaluate tumor-infiltrating CD8 + T cells in hepatocellular carcinoma patients using contrast-enhanced computed tomography[J]. Ann Surg Oncol, 2019, 26(13): 4537-4547. DOI: 10.1245/s10434-019-07815-9. |
| [31] |
SUN HC, RAO SX, XU B, et al. Predicting the efficacy of lenvatinib plus anti-PD-1 antibodies in unresectable hepatocellular carcinoma (uHCC) using radiomics features of tumors extracted from baseline MRI[J]. Ann Oncol, 2021, 32(Suppl 5): 948P.
|
| [32] |
OGSTON KN, MILLER ID, PAYNE S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: Prognostic significance and survival[J]. Breast, 2003, 12(5): 320-327. DOI: 10.1016/s0960-9776(03)00106-1. |
| [33] |
STEIN JE, LIPSON EJ, COTTRELL TR, et al. Pan-tumor pathologic scoring of response to PD-(L)1 blockade[J]. Clin Cancer Res, 2020, 26(3): 545-551. DOI: 10.1158/1078-0432.CCR-19-2379. |
| [34] |
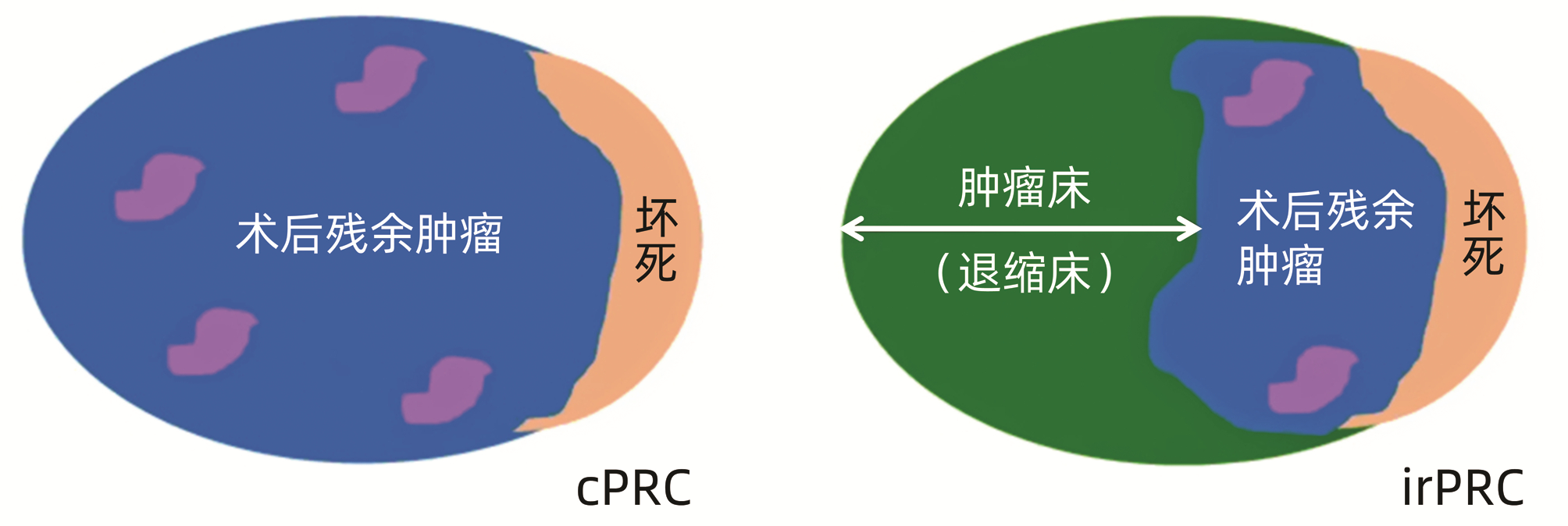
COTTRELL TR, THOMPSON ED, FORDE PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: A proposal for quantitative immune-related pathologic response criteria (irPRC)[J]. Ann Oncol, 2018, 29(8): 1853-1860. DOI: 10.1093/annonc/mdy218. |
| [35] |
LEON-MATEOS L, GARCIA-VELLOSO MJ, GARCÍA-FIGUEIRAS R, et al. A multidisciplinary consensus on the morphological and functional responses to immunotherapy treatment[J]. Clin Transl Oncol, 2021, 23(3): 434-449. DOI: 10.1007/s12094-020-02442-3. |
| [36] |
MARRON T, GUNASEKARAN G, TABRIZIAN P, et al. Neoadjuvant cemiplimab demonstrated complete pathological responses in hepatocelluler carcinoma[R]. 2021 AACR: CT182.
|
| [37] |
Alliance of Liver Cancer Conversion Therapy, Committee of Liver Cancer of the Chinese Anti-Cancer Association. Chinese expert consensus on conversion therapy in hepatocellular carcinoma (2021 edition)[J]. Chin J Dig Surg, 2021, 20(6): 600-616. DOI: 10.3760/cma.j.cn115610-20210512-00223. |
| [38] |
SONBOL MB, RIAZ IB, NAQVI S, et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: A systematic review and network meta-analysis[J]. JAMA Oncol, 2020, 6(12): e204930. DOI: 10.1001/jamaoncol.2020.4930. |
| [39] |
GALLE PR, KIM RD, SUNG MW, et al. Updated results of a phase Ib study of regorafenib (REG) plus pembrolizumab (PEMBRO) for first-line treatment of advanced hepatocellular carcinoma (HCC)[R]. EAMO Virtual 2020: 990P
|
| [40] |
CHENG AL, HSU C, CHAN SL, et al. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma[J]. J Hepatol, 2020, 72(2): 307-319. DOI: 10.1016/j.jhep.2019.09.025. |
| [41] |
STEIN S, HSU CH, LEE M, et al. Patterns of response to atezolizumab (atezo) + bevacizumab (bev) in hepatocellular carcinoma (HCC) from the Phase 1b GO30140 study[J]. Digital Liver Cancer Summit, 2021: 24.
|
| [42] |
BORCOMAN E, KANJANAPAN Y, CHAMPIAT S, et al. Novel patterns of response under immunotherapy[J]. Ann Oncol, 2019, 30(3): 385-396. DOI: 10.1093/annonc/mdz003. |
| [43] |
SPAGNOLO F, BOUTROS A, CECCHI F, et al. Treatment beyond progression with anti-PD-1/PD-L1 based regimens in advanced solid tumors: A systematic review[J]. BMC Cancer, 2021, 21(1): 425. DOI: 10.1186/s12885-021-08165-0. |
| [44] |
Chinese Society of Liver Cancer, Chinese Anti-Cancer Association. Chinese expert consensus on multidisciplinary treatment of liver cancer[J]. J Clin Hepatol, 2021, 37(2): 278-285. DOI: 10.3969/j.issn.1001-5256.2021.02.008. |








 DownLoad:
DownLoad: