| [1] |
BISSELL DM, ANDERSON KE, BONKOVSKY HL. Porphyria[J]. N Engl J Med, 2017, 377(9): 862-872. DOI: 10.1056/NEJMra1608634. |
| [2] |
BONKOVSKY HL, MADDUKURI VC, YAZICI C, et al. Acute porphyrias in the USA: Features of 108 subjects from porphyrias consortium[J]. Am J Med, 2014, 127(12): 1233-1241. DOI: 10.1016/j.amjmed.2014.06.036. |
| [3] |
CHEN Y, LI XQ. An excerpt of acute hepatic porphyrias: Recommendations for evaluation and long term management of the NCATS Rare Diseases Clinical lksearch Network[J]. J Clin Hepatol, 2017, 33(11): 2083-2086. DOI: 10.3969/j.issn.1001-5256.2017.11.006. |
| [4] |
FONTANELLAS A, ÁVILA MA, ANDERSON KE, et al. Current and innovative emerging therapies for porphyrias with hepatic involvement[J]. J Hepatol, 2019, 71(2): 422-433. DOI: 10.1016/j.jhep.2019.05.003. |
| [5] |
WANG B, RUDNICK S, CENGIA B, et al. Acute hepatic porphyrias: Review and recent progress[J]. Hepatol Commun, 2019, 3(2): 193-206. DOI: 10.1002/hep4.1297. |
| [6] |
GOMÁ-GARCÉS E, PÉREZ-GÓMEZ MV, ORTÍZ A. Givosiran for acute intermittent porphyria[J]. N Engl J Med, 2020, 383(20): 1989. DOI: 10.1056/NEJMc2026458. |
| [7] |
BUNG N, ROY A, PRIYAKUMAR UD, et al. Computational modeling of the catalytic mechanism of hydroxymethylbilane synthase[J]. Phys Chem Chem Phys, 2019, 21(15): 7932-7940. DOI: 10.1039/c9cp00196d. |
| [8] |
RAMANUJAM VS, ANDERSON KE. Porphyria diagnostics-part 1: A brief overview of the porphyrias[J]. Curr Protoc Hum Genet, 2015, 86: 17.20.1-17.20.26. DOI: 10.1002/0471142905.hg1720s86. |
| [9] |
BALWANI M, WANG B, ANDERSON KE, et al. Acute hepatic porphyrias: Recommendations for evaluation and long-term management[J]. Hepatology, 2017, 66(4): 1314-1322. DOI: 10.1002/hep.29313. |
| [10] |
BISSELL DM, LAI JC, MEISTER RK, et al. Role of delta-aminolevulinic acid in the symptoms of acute porphyria[J]. Am J Med, 2015, 128(3): 313-317. DOI: 10.1016/j.amjmed.2014.10.026. |
| [11] |
OLIVERI LM, DAVIO C, BATLLE AM, et al. ALAS1 gene expression is down-regulated by Akt-mediated phosphorylation and nuclear exclusion of FOXO1 by vanadate in diabetic mice[J]. Biochem J, 2012, 442(2): 303-310. DOI: 10.1042/BJ20111005. |
| [12] |
ANDERSON KE. Acute hepatic porphyrias: Current diagnosis & management[J]. Mol Genet Metab, 2019, 128(3): 219-227. DOI: 10.1016/j.ymgme.2019.07.002. |
| [13] |
SCASSA ME, VARONE CL, MONTERO L, et al. Insulin inhibits delta-aminolevulinate synthase gene expression in rat hepatocytes and human hepatoma cells[J]. Exp Cell Res, 1998, 244(2): 460-469. DOI: 10.1006/excr.1998.4206. |
| [14] |
FONTANELLAS A, ÁVILA MA, BERRAONDO P. Emerging therapies for acute intermittent porphyria[J]. Expert Rev Mol Med, 2016, 18: e17. DOI: 10.1017/erm.2016.18. |
| [15] |
ZHANG J, HU Y, ZHENG J, et al. Treatment of acute intermittent porphyria during pregnancy and posterior reversible encephalopathy syndrome after delivery: A case report[J]. Exp Ther Med, 2017, 14(6): 5554-5556. DOI: 10.3892/etm.2017.5212. |
| [16] |
YARRA P, FAUST D, BENNETT M, et al. Benefits of prophylactic heme therapy in severe acute intermittent porphyria[J]. Mol Genet Metab Rep, 2019, 19: 100450. DOI: 10.1016/j.ymgmr.2019.01.002. |
| [17] |
BLAYLOCK B, EPSTEIN J, STICKLER P. Real-world annualized healthcare utilization and expenditures among insured US patients with acute intermittent porphyria (AIP) treated with hemin[J]. J Med Econ, 2020, 23(6): 537-545. DOI: 10.1080/13696998.2020.1724118. |
| [18] |
WILLANDT B, LANGENDONK JG, BIERMANN K, et al. Liver fibrosis associated with iron accumulation due to long-term heme-arginate treatment in acute intermittent porphyria: A case series[J]. JIMD Rep, 2016, 25: 77-81. DOI: 10.1007/8904_2015_458. |
| [19] |
SCHMITT C, LENGLET H, YU A, et al. Recurrent attacks of acute hepatic porphyria: Major role of the chronic inflammatory response in the liver[J]. J Intern Med, 2018, 284(1): 78-91. DOI: 10.1111/joim.12750. |
| [20] |
PISCHIK E, KAUPPINEN R. An update of clinical management of acute intermittent porphyria[J]. Appl Clin Genet, 2015, 8: 201-214. DOI: 10.2147/TACG.S48605. |
| [21] |
Study Group of Red Blood Cell Disease (Anemia), Chinese Society of Hematology, Chinese Medical Association. Expert consensus on diagnosis and treatment of porphyria in China(2020)[J]. Natl Med J China, 2020, 100(14): 1051-1056. DOI: 10.3760/cma.j.cn112137-20200219-00349. |
| [22] |
CARDENAS JL, GUERRERO C. Acute intermittent porphyria: General aspects with focus on pain[J]. Curr Med Res Opin, 2018, 34(7): 1309-1315. DOI: 10.1080/03007995.2018.1435521. |
| [23] |
GONZALEZ-MOSQUERA LF, SONTHALIA S. Acute Intermittent Porphyria[A]. Treasure Island (FL), 2021.
|
| [24] |
GOUYA L, VENTURA P, BALWANI M, et al. EXPLORE: A prospective, multinational, natural history study of patients with acute hepatic porphyria with recurrent attacks[J]. Hepatology, 2020, 71(5): 1546-1558. DOI: 10.1002/hep.30936. |
| [25] |
SCHULENBURG-BRAND D, GARDINER T, GUPPY S, et al. An audit of the use of gonadorelin analogues to prevent recurrent acute symptoms in patients with acute porphyria in the United Kingdom[J]. JIMD Rep, 2017, 36: 99-107. DOI: 10.1007/8904_2017_2. |
| [26] |
MARSDEN JT, GUPPY S, STEIN P, et al. Audit of the use of regular haem arginate infusions in patients with acute porphyria to prevent recurrent symptoms[J]. JIMD Rep, 2015, 22: 57-65. DOI: 10.1007/8904_2015_411. |
| [27] |
SINGAL AK, PARKER C, BOWDEN C, et al. Liver transplantation in the management of porphyria[J]. Hepatology, 2014, 60(3): 1082-1089. DOI: 10.1002/hep.27086. |
| [28] |
LISSING M, NOWAK G, ADAM R, et al. Liver transplantation for acute intermittent porphyria[J]. Liver Transpl, 2021, 27(4): 491-501. DOI: 10.1002/lt.25959. |
| [29] |
D'AVOLA D, LÓPEZ-FRANCO E, SANGRO B, et al. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria[J]. J Hepatol, 2016, 65(4): 776-783. DOI: 10.1016/j.jhep.2016.05.012. |
| [30] |
NAULT JC, DATTA S, IMBEAUD S, et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas[J]. Nat Genet, 2015, 47(10): 1187-1193. DOI: 10.1038/ng.3389. |
| [31] |
SERRANO-MENDIOROZ I, SAMPEDRO A, SERNA N, et al. Bioengineered PBGD variant improves the therapeutic index of gene therapy vectors for acute intermittent porphyria[J]. Hum Mol Genet, 2018, 27(21): 3688-3696. DOI: 10.1093/hmg/ddy283. |
| [32] |
SERRANO-MENDIOROZ I, SAMPEDRO A, ALEGRE M, et al. An Inducible Promoter responsive to different porphyrinogenic stimuli improves gene therapy vectors for acute intermittent porphyria[J]. Hum Gene Ther, 2018, 29(4): 480-491. DOI: 10.1089/hum.2017.056. |
| [33] |
JIANG L, BERRAONDO P, JERICÓ D, et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria[J]. Nat Med, 2018, 24(12): 1899-1909. DOI: 10.1038/s41591-018-0199-z. |
| [34] |
PARRA-GUILLEN ZP, FONTANELLAS A, JIANG L, et al. Disease pharmacokinetic-pharmacodynamic modelling in acute intermittent porphyria to support the development of mRNA-based therapies[J]. Br J Pharmacol, 2020, 177(14): 3168-3182. DOI: 10.1111/bph.15040. |
| [35] |
de PAULA BRANDÃO PR, TITZE-DE-ALMEIDA SS, TITZE-DE-ALMEIDA R. Leading RNA interference therapeutics part 2: Silencing delta-aminolevulinic acid synthase 1, with a focus on givosiran[J]. Mol Diagn Ther, 2020, 24(1): 61-68. DOI: 10.1007/s40291-019-00438-6. |
| [36] |
SARDH E, HARPER P, BALWANI M, et al. Phase 1 trial of an RNA interference therapy for acute intermittent porphyria[J]. N Engl J Med, 2019, 380(6): 549-558. DOI: 10.1056/NEJMoa1807838. |
| [37] |
|
| [38] |
BALWANI M, SARDH E, VENTURA P, et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria[J]. N Engl J Med, 2020, 382(24): 2289-2301. DOI: 10.1056/NEJMoa1913147. |
| [39] |
AGARWAL S, SIMON AR, GOEL V, et al. Pharmacokinetics and pharmacodynamics of the small interfering ribonucleic acid, givosiran, in patients with acute hepatic porphyria[J]. Clin Pharmacol Ther, 2020, 108(1): 63-72. DOI: 10.1002/cpt.1802. |
| [40] |
RAY K. Interfering with acute intermittent porphyria[J]. Nat Rev Gastroenterol Hepatol, 2020, 17(8): 452. DOI: 10.1038/s41575-020-0335-3. |
| [41] |
BUSTAD HJ, TOSKA K, SCHMITT C, et al. A pharmacological chaperone therapy for acute intermittent porphyria[J]. Mol Ther, 2020, 28(2): 677-689. DOI: 10.1016/j.ymthe.2019.11.010. |



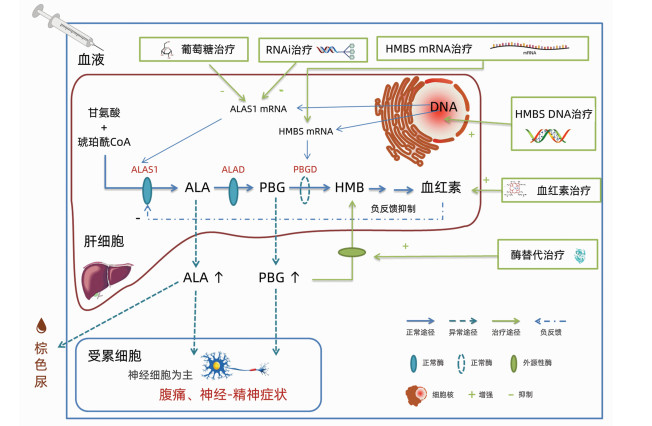




 DownLoad:
DownLoad: