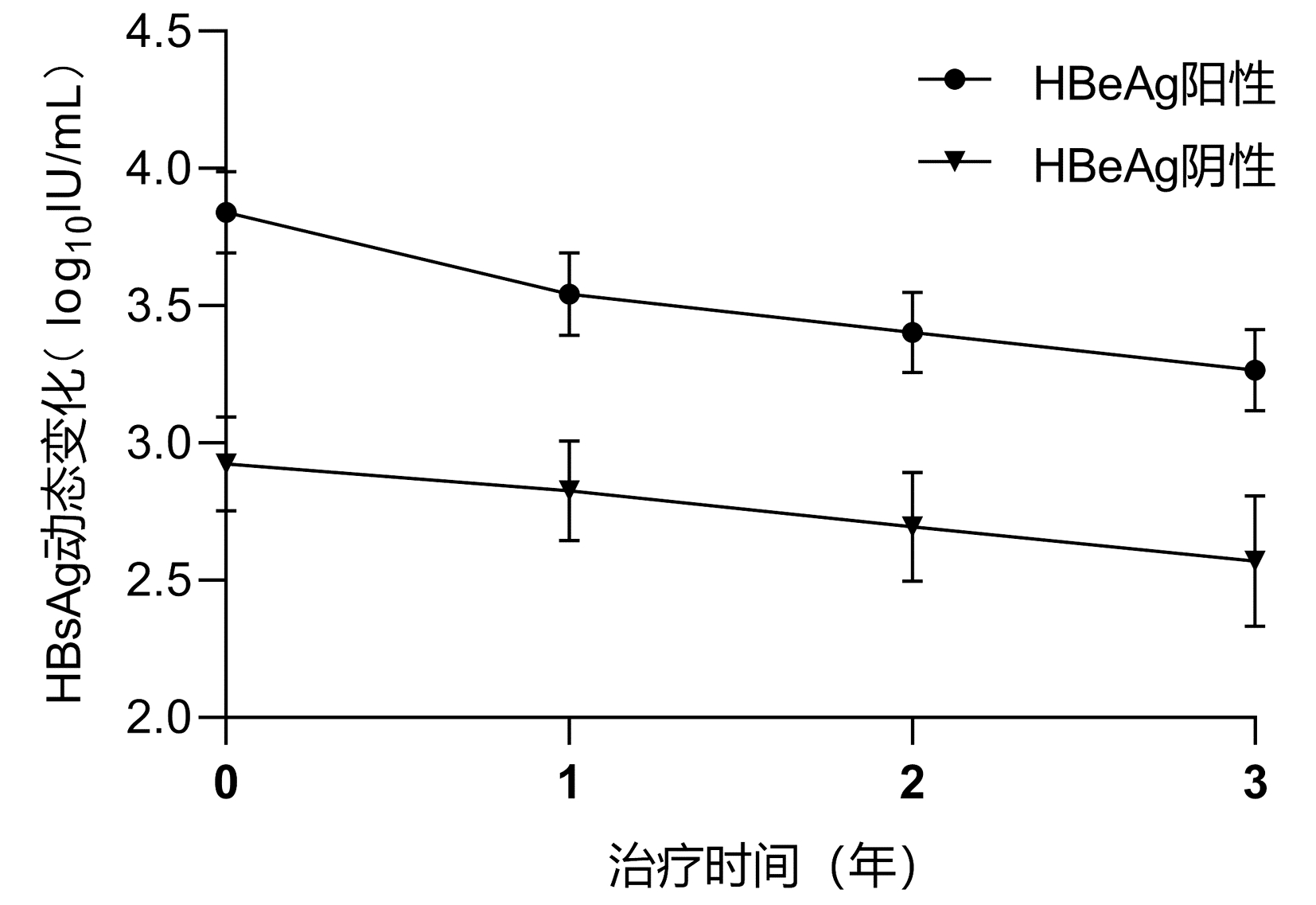
| [1] |
Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. |
| [2] |
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. |
| [3] |
NA JH, KIM JH, CHOE WH, et al. Changes in the hepatitis B surface antigen level according to the HBeAg status and drug used in long-term nucleos(t)ide analog-treated chronic hepatitis B patients[J]. Korean J Gastroenterol, 2021, 77(6): 285-293. DOI: 10.4166/kjg.2021.043. |
| [4] |
WU FP, LI MX, WANG YK, et al. Dynamic changes of HBsAg and hepatitis B virus DNA in chronic hepatitis B patients after entecavir monotherapy for 5 years[J]. J Clin Hepatol, 2021, 37(5): 1047-1052. DOI: 10.3969/j.issn.1001-5256.2021.05.015. |
| [5] |
RIVEIRO-BARCIELA M, TABERNERO D, CALLEJA JL, et al. Effectiveness and safety of entecavir or tenofovir in a spanish cohort of chronic hepatitis B patients: Validation of the Page-B Score to predict hepatocellular carcinoma[J]. Dig Dis Sci, 2017, 62(3): 784-793. DOI: 10.1007/s10620-017-4448-7. |
| [6] |
ZHANG Q, CAI DC, HU P, et al. Low-level viremia in nucleoside analog-treated chronic hepatitis B patients[J]. Chin Med J (Engl), 2021, 134(23): 2810-2817. DOI: 10.1097/CM9.0000000000001793. |
| [7] |
LU FM, FENG B, ZHENG SJ, et al. Current status of the research on low-level viremia in chronic hepatitis B patients receiving nucleos(t)ide analogues[J]. J Clin Hepatol, 2021, 37(6): 1268-1274. DOI: 10.3969/j.issn.1001-5256.2021.06.007. |
| [8] |
TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. |
| [9] |
BOYD A, LACOMBE K, LAVOCAT F, et al. Decay of ccc-DNA marks persistence of intrahepatic viral DNA synthesis under tenofovir in HIV-HBV co-infected patients[J]. J Hepatol, 2016, 65(4): 683-691. DOI: 10.1016/j.jhep.2016.05.014. |
| [10] |
PARK ES, LEE AR, KIM DH, et al. Identification of a quadruple mutation that confers tenofovir resistance in chronic hepatitis B patients[J]. J Hepatol, 2019, 70(6): 1093-1102. DOI: 10.1016/j.jhep.2019.02.006. |
| [11] |
MOKAYA J, MCNAUGHTON AL, BESTER PA, et al. Hepatitis B virus resistance to tenofovir: fact or fiction? A systematic literature review and structural analysis of drug resistance mechanisms[J]. Wellcome Open Res, 2020, 5: 151. DOI: 10.12688/wellcomeopenres.15992.1. |
| [12] |
DI BISCEGLIE AM, KING WC, LISKER-MELMAN M, et al. Age, race and viral genotype are associated with the prevalence of hepatitis B e antigen in children and adults with chronic hepatitis B[J]. J Viral Hepat, 2019, 26(7): 856-865. DOI: 10.1111/jvh.13104. |
| [13] |
CHAN HL, WONG VW, WONG GL, et al. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B[J]. Hepatology, 2010, 52(4): 1232-1241. DOI: 10.1002/hep.23803. |
| [14] |
CELIK M, ARABUL M, CEKIÇ C, et al. Clinical utility of hepatitis B surface antigen levels during the natural history and treatment of chronic hepatitis B infection[J]. Prz Gastroenterol, 2014, 9(3): 164-167. DOI: 10.5114/pg.2014.43758. |
| [15] |
ZOUTENDIJK R, HANSEN BE, VAN VUUREN AJ, et al. Serum HBsAg decline during long-term potent nucleos(t)ide analogue therapy for chronic hepatitis B and prediction of HBsAg loss[J]. J Infect Dis, 2011, 204(3): 415-418. DOI: 10.1093/infdis/jir282. |
| [16] |
YU Y, HOU J, OMATA M, et al. Loss of HBsAg and antiviral treatment: from basics to clinical significance[J]. Hepatol Int, 2014, 8(1): 39-54. DOI: 10.1007/s12072-013-9495-3. |
| [17] |
SETO WK, LIU K, WONG DK, et al. Patterns of hepatitis B surface antigen decline and HBV DNA suppression in Asian treatment-experienced chronic hepatitis B patients after three years of tenofovir treatment[J]. J Hepatol, 2013, 59(4): 709-716. DOI: 10.1016/j.jhep.2013.06.007. |
| [18] |
MANESIS EK, PAPATHEODORIDIS GV, TINIAKOS DG, et al. Hepatitis B surface antigen: relation to hepatitis B replication parameters in HBeAg-negative chronic hepatitis B[J]. J Hepatol, 2011, 55(1): 61-68. DOI: 10.1016/j.jhep.2010.10.027. |
| [19] |
LEVRERO M, POLLICINO T, PETERSEN J, et al. Control of cccDNA function in hepatitis B virus infection[J]. J Hepatol, 2009, 51(3): 581-592. DOI: 10.1016/j.jhep.2009.05.022. |
| [20] |
Chinese Society of Infectious Disease, Chinese Society of Hepatology, Chinese Medical Association. The expert consensus on clinical cure (functional cure) of chronic hepatitis B[J]. J Clin Hepatol, 2019, 35(8): 1693-1701. DOI: 10.3969/j.issn.1001-5256.2019.08.008. |
| [21] |
HUANG J, ZHANG K, CHEN W, et al. Switching to PegIFNα-2b leads to HBsAg loss in patients with low HBsAg levels and HBV DNA suppressed by NAs[J]. Sci Rep, 2017, 7(1): 13383. DOI: 10.1038/s41598-017-13747-9. |
| [22] |
WU FP, YANG Y, LI M, et al. Add-on pegylated interferon augments hepatitis B surface antigen clearance vs continuous nucleos(t)ide analog monotherapy in Chinese patients with chronic hepatitis B and hepatitis B surface antigen ≤ 1500 IU/mL: An observational study[J]. World J Gastroenterol, 2020, 26(13): 1525-1539. DOI: 10.3748/wjg.v26.i13.1525. |
| [23] |
LI H, XU WT, DENG BC, et al. Research progress of nucleoside analogues combined with peginterferon in functional cure of chronic hepatitis B[J]. Chin J Med Offic, 2022, 50(9): 890-893. DOI: 10.16680/j.1671-3826.2022.09.04. |



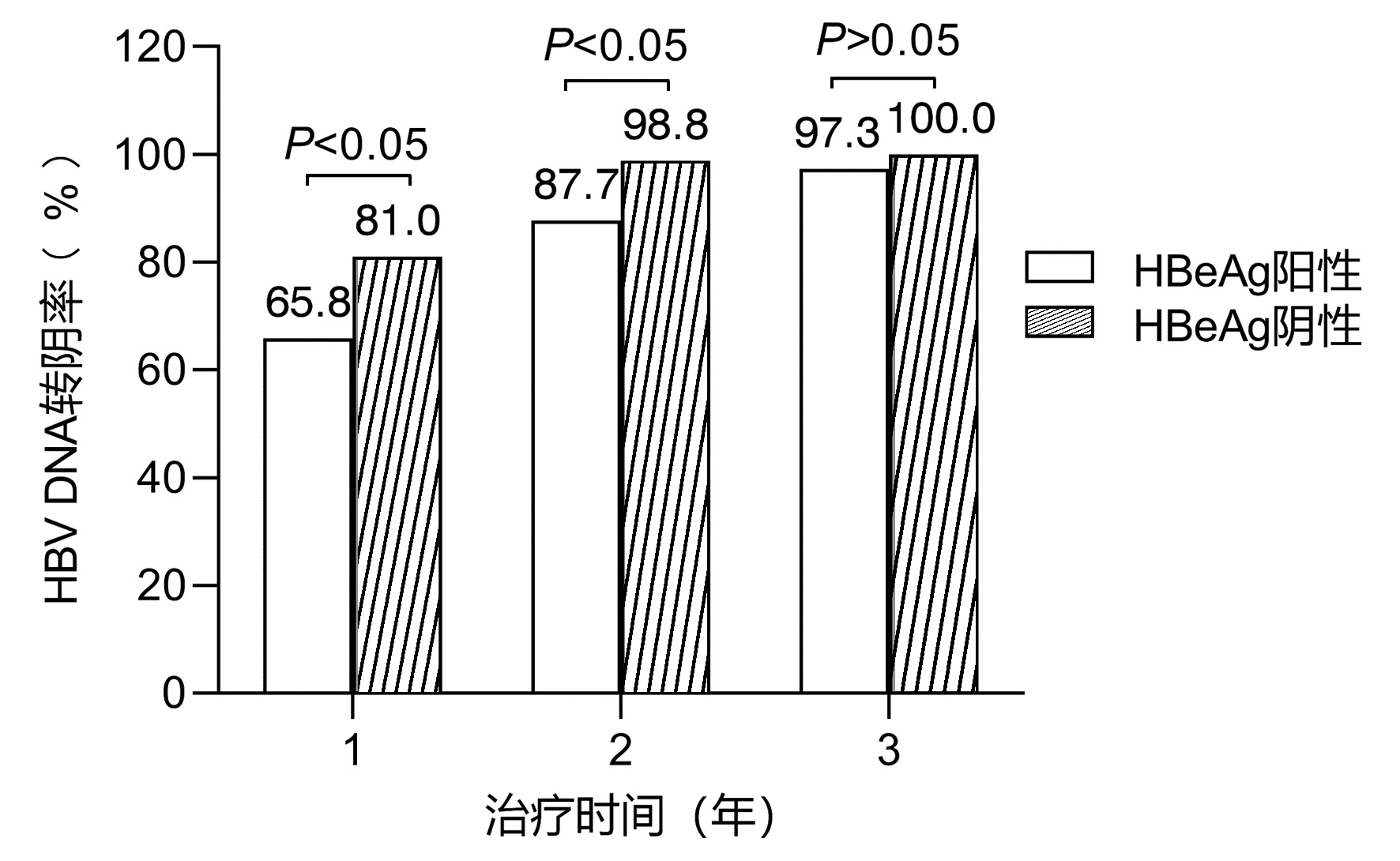




 DownLoad:
DownLoad: