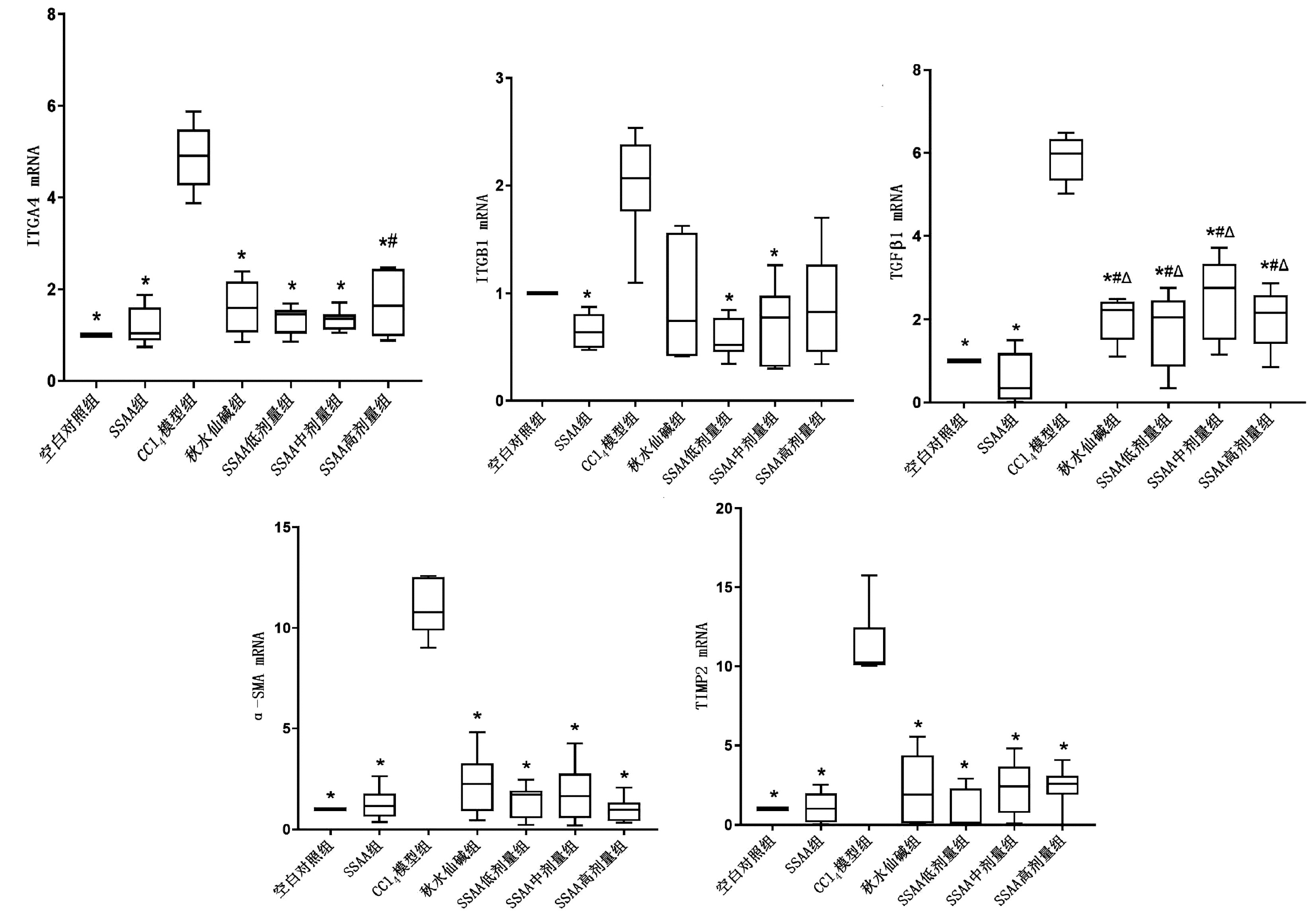
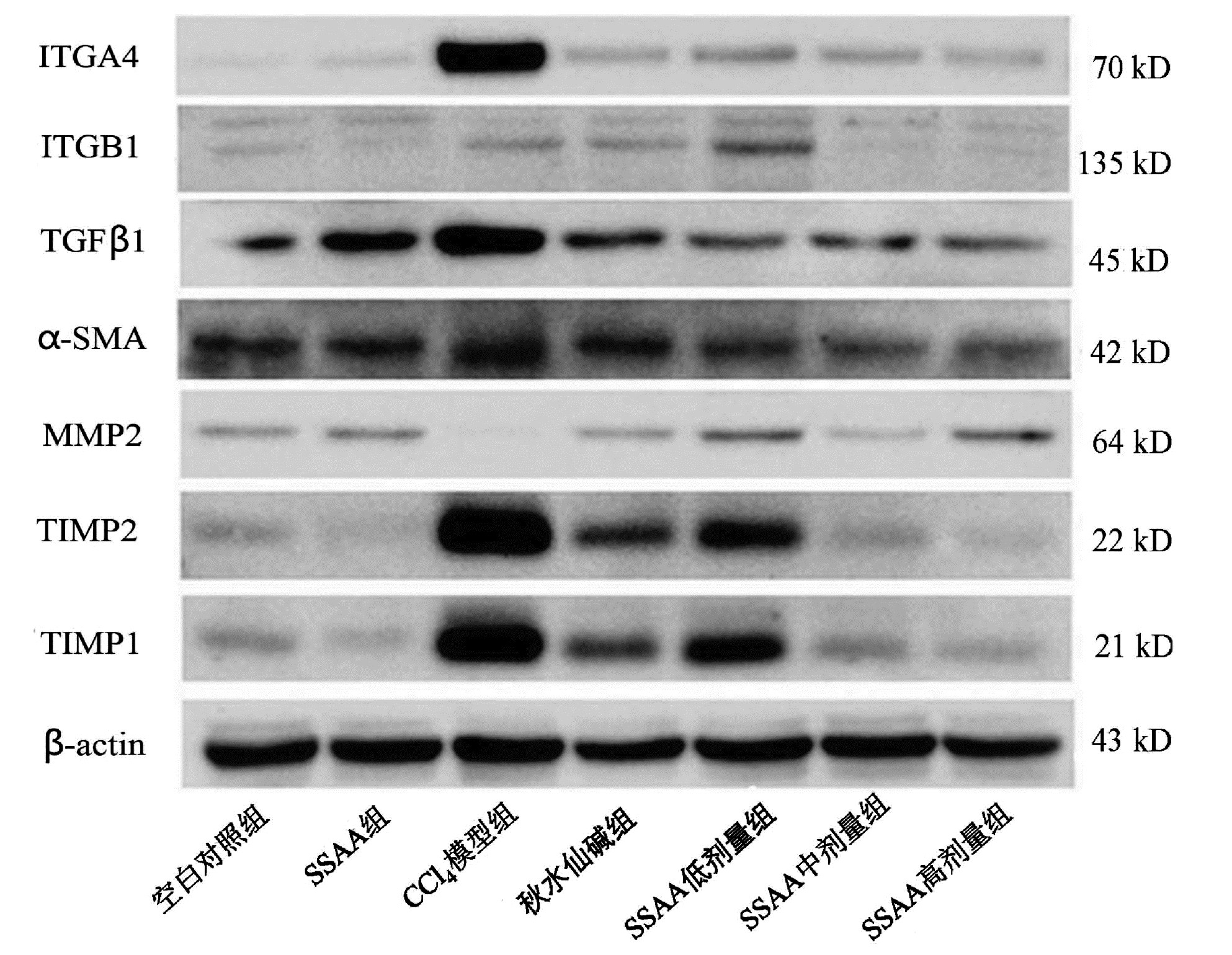
| [1] |
LI Y, PU S, LIU Q, et al. An integrin-based nanoparticle that targets activated hepatic stellate cells and alleviates liver fibrosis[J]. J Control Release, 2019, 303: 77-90. DOI: 10.1016/j.jconrel.2019.04.022. |
| [2] |
PULKKA OP, MPINDI JP, TYNNINEN O, et al. Clinical relevance of integrin alpha 4 in gastrointestinal stromal tumours[J]. J Cell Mol Med, 2018, 22(4): 2220-2230. DOI: 10.1111/jcmm.13502. |
| [3] |
HINTERMANN E, CHRISTEN U. The many roles of cell adhesion molecules in hepatic fibrosis[J]. Cells, 2019, 8(12): 1503. DOI: 10.3390/cells8121503. |
| [4] |
SFERRA R, VETUSCHI A, POMPILI S, et al. Expression of pro-fibrotic and anti-fibrotic molecules in dimethylnitrosamine-induced hepatic fibrosis[J]. Pathol Res Pract, 2017, 213(1): 58-65. DOI: 10.1016/j.prp.2016.11.004. |
| [5] |
SHAO T, CHEN Z, BELOV V, et al. [18F]-Alfatide PET imaging of integrin αvβ3 for the non-invasive quantification of liver fibrosis[J]. J Hepatol, 2020, 73(1): 161-169. DOI: 10.1016/j.jhep.2020.02.018. |
| [6] |
BERNSMEIER C, van DER MERWE S, PÉRIANIN A. Innate immune cells in cirrhosis[J]. J Hepatol, 2020, 73(1): 186-201. DOI: 10.1016/j.jhep.2020.03.027. |
| [7] |
SHAO MY, LAI Y. Effects of Ganlong Capsules combined with silibinin on the expression of Col-Ⅰ and TIMP-1 genes in liver tissue of hepatic fibrosis rats[J]. Advances in Veterinary Med, 2018, 39(10): 50-55. DOI: 10.16437/j.cnki.1007-5038.2018.10.010. |
| [8] |
HU YF. Efficacy and survival analysis of plasma exchange in patients with acute-on-chronic liver failure[D]. Zhengzhou: Zhengzhou University, 2020.
呼怡菲. 血浆置换治疗慢加急性肝衰竭患者的疗效及生存分析[D]. 郑州: 郑州大学, 2020.
|
| [9] |
TSUCHIDA T, FRIEDMAN SL. Mechanisms of hepatic stellate cell activation[J]. Nat Rev Gastroenterol Hepatol, 2017, 14(7): 397-411. DOI: 10.1038/nrgastro.2017.38. |
| [10] |
LACHOWSKI D, CORTES E, RICE A, et al. Matrix stiffness modulates the activity of MMP-9 and TIMP-1 in hepatic stellate cells to perpetuate fibrosis[J]. Sci Rep, 2019, 9(1): 7299. DOI: 10.1038/s41598-019-43759-6. |
| [11] |
ZHOU J, LI R, ZHANG J, et al. Targeting interstitial myofibroblast-expressed integrin αvβ3 alleviates renal fibrosis[J]. Mol Pharm, 2021, 18(3): 1373-1385. DOI: 10.1021/acs.molpharmaceut.0c01182. |
| [12] |
HUANG YZ, ZHAO L, ZHU Y, et al. Interrupting TGF-β1/CCN2/integrin-α5β1 signaling alleviates high mechanical-stress caused chondrocyte fibrosis[J]. Eur Rev Med Pharmacol Sci, 2021, 25(3): 1233-1241. DOI: 10.26355/eurrev_202102_24827. |
| [13] |
STRUDWICK XL, ADAMS DH, PYNE NT, et al. Systemic delivery of anti-integrin αL antibodies reduces early macrophage recruitment, inflammation, and scar formation in murine burn wounds[J]. Adv Wound Care (New Rochelle), 2020, 9(12): 637-648. DOI: 10.1089/wound.2019.1035. |
| [14] |
LIU W, SUN T, WANG Y. Integrin αvβ6 mediates epithelial-mesenchymal transition in human bronchial epithelial cells induced by lipopolysaccharides of Pseudomonas aeruginosa via TGF-β1-Smad2/3 signaling pathway[J]. Folia Microbiol (Praha), 2020, 65(2): 329-338. DOI: 10.1007/s12223-019-00728-w. |
| [15] |
BONUS M, HÄUSSINGER D, GOHLKE H. Liver cell hydration and integrin signaling[J]. Biol Chem, 2021, 402(9): 1033-1045. DOI: 10.1515/hsz-2021-0193. |
| [16] |
HIGHT-WARBURTON W, PARSONS M. Regulation of cell migration by α4 and α9 integrins[J]. Biochem J, 2019, 476(4): 705-718. DOI: 10.1042/BCJ20180415. |
| [17] |
KUMMER C, PETRICH BG, ROSE DM, et al. A small molecule that inhibits the interaction of paxillin and alpha 4 integrin inhibits accumulation of mononuclear leukocytes at a site of inflammation[J]. J Biol Chem, 2010, 285(13): 9462-9469. DOI: 10.1074/jbc.M109.066993. |
| [18] |
DAMMES N, GOLDSMITH M, RAMISHETTI S, et al. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics[J]. Nat Nanotechnol, 2021, 16(9): 1030-1038. DOI: 10.1038/s41565-021-00928-x. |
| [19] |
RAI RP, LIU Y, IYER SS, et al. Blocking integrin α4β7-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis[J]. J Hepatol, 2020, 73(5): 1013-1022. DOI: 10.1016/j.jhep.2020.05.047. |








 DownLoad:
DownLoad: