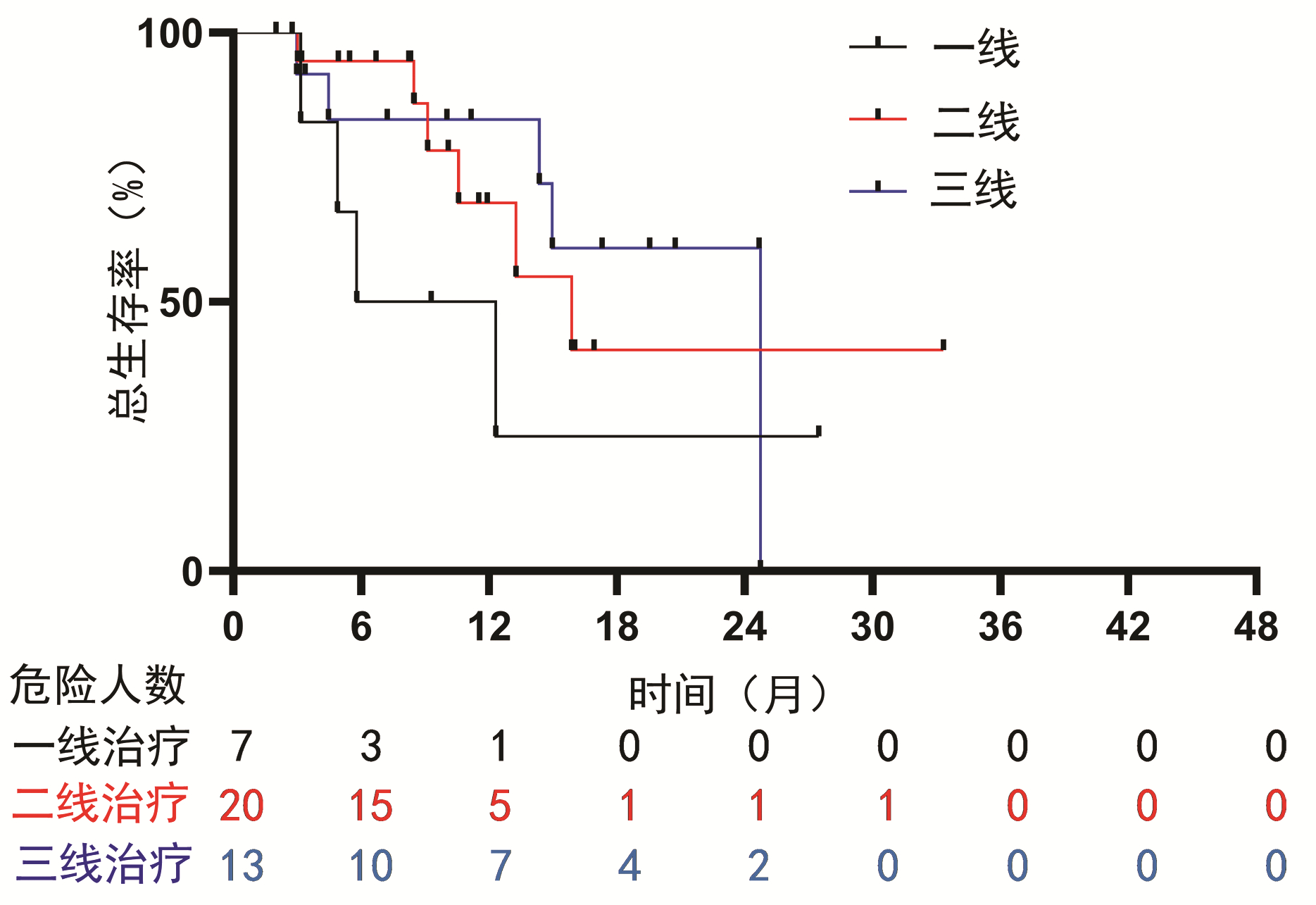
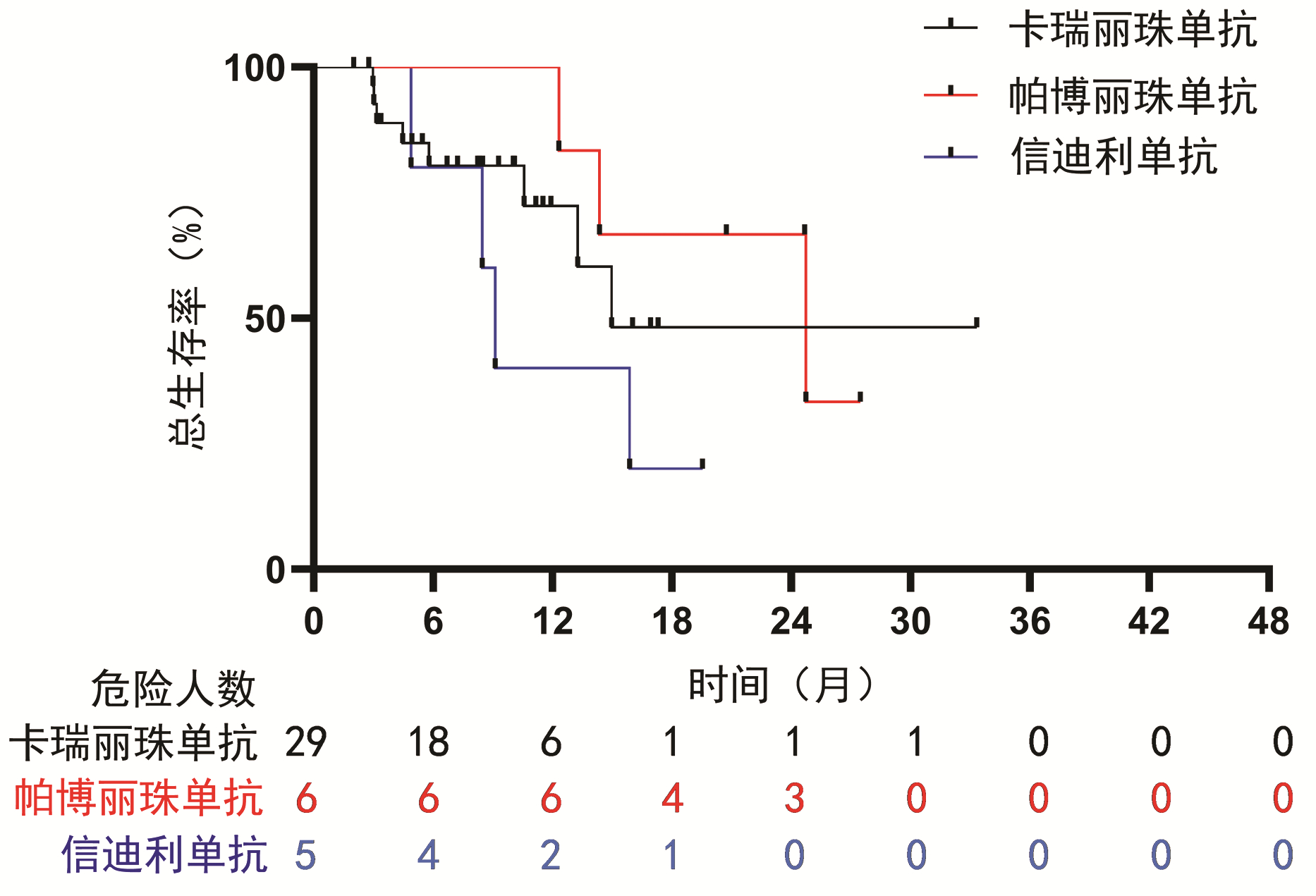
| [1] |
VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380(15): 1450-1462. DOI: 10.1056/NEJMra1713263. |
| [2] |
LLOVET JM, KELLEY RK, VILLANUEVA A, et al. Hepatocellular carcinoma[J]. Nat Rev Dis Primers, 2021, 7(1): 6. DOI: 10.1038/s41572-020-00240-3. |
| [3] |
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2012, 56(4): 908-943. DOI: 10.1016/j.jhep.2011.12.001. |
| [4] |
LLOVET JM, ZUCMAN-ROSSI J, PIKARSKY E, et al. Hepatocellular carcinoma[J]. Nat Rev Dis Primers, 2016, 2: 16018. DOI: 10.1038/nrdp.2016.18. |
| [5] |
|
| [6] |
VOGEL A, CERVANTES A, CHAU I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2018, 29(Suppl 4): iv238-iv255. DOI: 10.1093/annonc/mdy308. |
| [7] |
HARTKE J, JOHNSON M, GHABRIL M. The diagnosis and treatment of hepatocellular carcinoma[J]. Semin Diagn Pathol, 2017, 34(2): 153-159. DOI: 10.1053/j.semdp.2016.12.011. |
| [8] |
LLOVET JM, REAL MI, MONTAÑA X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial[J]. Lancet, 2002, 359(9319): 1734-1739. DOI: 10.1016/S0140-6736(02)08649-X. |
| [9] |
LO CM, NGAN H, TSO WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma[J]. Hepatology, 2002, 35(5): 1164-1171. DOI: 10.1053/jhep.2002.33156. |
| [10] |
KIRSTEIN MM, VOIGTLÄNDER T, SCHWEITZER N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease[J]. United European Gastroenterol J, 2018, 6(2): 238-246. DOI: 10.1177/2050640617716597. |
| [11] |
RAOUL JL, FORNER A, BOLONDI L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence[J]. Cancer Treat Rev, 2019, 72: 28-36. DOI: 10.1016/j.ctrv.2018.11.002. |
| [12] |
WANG Q, XIA D, BAI W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study[J]. J Hepatol, 2019, 70(5): 893-903. DOI: 10.1016/j.jhep.2019.01.013. |
| [13] |
KUDO M, UESHIMA K, IKEDA M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial[J]. Gut, 2020, 69(8): 1492-1501. DOI: 10.1136/gutjnl-2019-318934. |
| [14] |
PRIETO J, MELERO I, SANGRO B. Immunological landscape and immunotherapy of hepatocellular carcinoma[J]. Nat Rev Gastroenterol Hepatol, 2015, 12(12): 681-700. DOI: 10.1038/nrgastro.2015.173. |
| [15] |
MONTASSER A, BEAUFRÈRE A, CAUCHY F, et al. Transarterial chemoembolisation enhances programmed death-1 and programmed death-ligand 1 expression in hepatocellular carcinoma[J]. Histopathology, 2021, 79(1): 36-46. DOI: 10.1111/his.14317. |
| [16] |
JOHN BV, SCHWARTZ K, LEVY C, et al. Impact of obeticholic acid exposure on decompensation and mortality in primary biliary cholangitis and cirrhosis[J]. Hepatol Commun, 2021, 5(8): 1426-1436. DOI: 10.1002/hep4.1720. |
| [17] |
SUZUKI H, MORI M, KAWAGUCHI C, et al. Serum vascular endothelial growth factor in the course of transcatheter arterial embolization of hepatocellular carcinoma[J]. Int J Oncol, 1999, 14(6): 1087-1090. DOI: 10.3892/ijo.14.6.1087. |
| [18] |
NOMAN MZ, DESANTIS G, JANJI B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation[J]. J Exp Med, 2014, 211(5): 781-790. DOI: 10.1084/jem.20131916. |
| [19] |
ZHENG L, FANG S, WU F, et al. Efficacy and safety of TACE combined with sorafenib plus immune checkpoint inhibitors for the treatment of intermediate and advanced TACE-refractory hepatocellular carcinoma: A retrospective Study[J]. Front Mol Biosci, 2021, 7: 609322. DOI: 10.3389/fmolb.2020.609322. |
| [20] |
LENCIONI R, DE BAERE T, SOULEN MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data[J]. Hepatology, 2016, 64(1): 106-116. DOI: 10.1002/hep.28453. |
| [21] |
HEIMBACH JK, KULIK LM, FINN RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma[J]. Hepatology, 2018, 67(1): 358-380. DOI: 10.1002/hep.29086. |
| [22] |
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. |
| [23] |
Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. |
| [24] |
KUDO M. Targeted and immune therapies for hepatocellular carcinoma: Predictions for 2019 and beyond[J]. World J Gastroenterol, 2019, 25(7): 789-807. DOI: 10.3748/wjg.v25.i7.789. |
| [25] |
FINN RS, QIN S, IKEDA M, et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20): 1894-1905. DOI: 10.1056/NEJMoa1915745. |



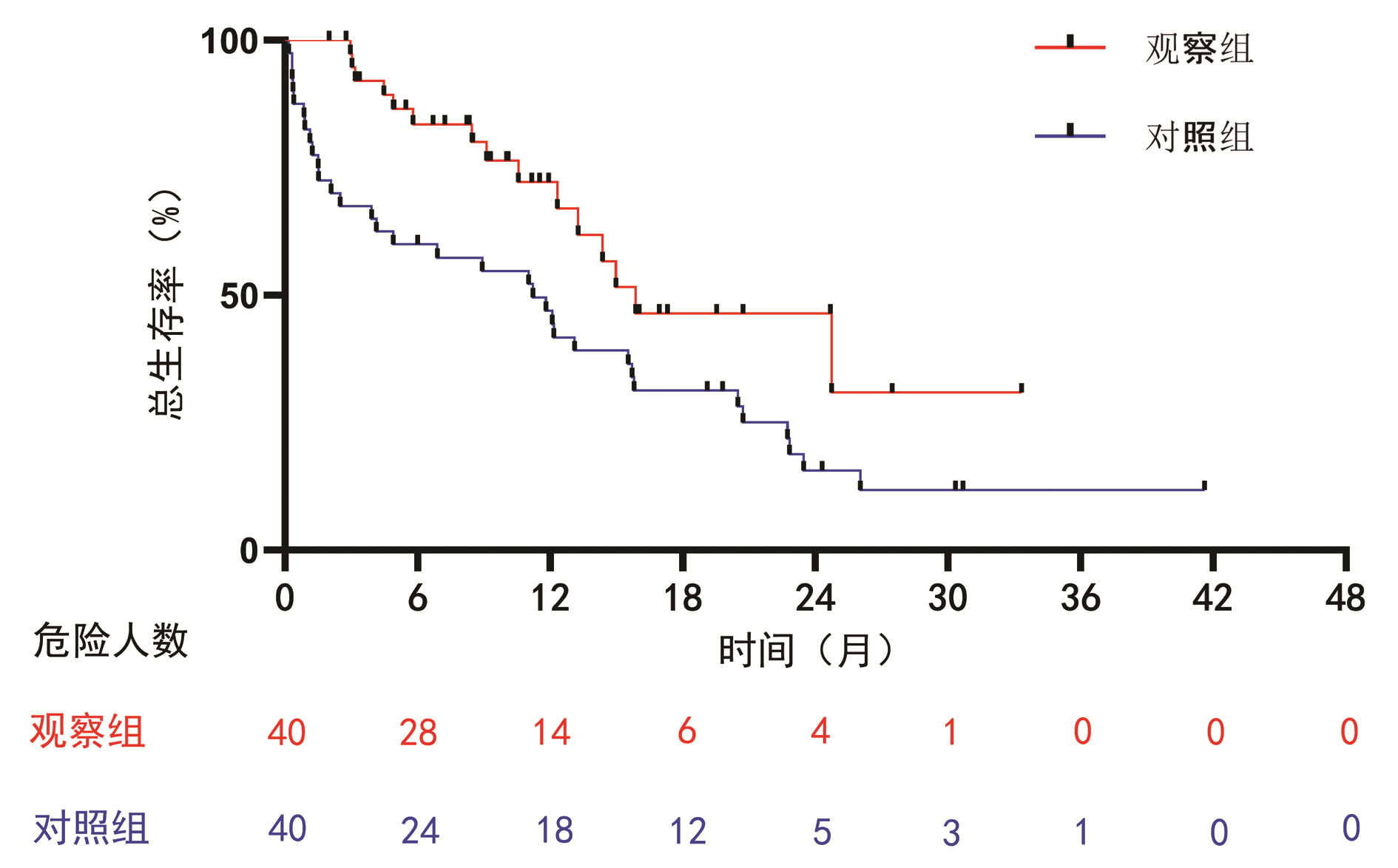




 DownLoad:
DownLoad: