| [1] |
Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C(2019 version)[J]. J Clin Hepatol, 2019, 35(12): 2670-2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008. |
| [2] |
CHEN HY, KANG Q, LUO H, et al. Virological response to direct-acting antiviral therapy and changes in liver fibrosis indices in chronic hepatitis C patients with different alanine aminotransferase and aspartate aminotransferase levels in a real-world setting[J]. J Clin Hepatol, 2021, 37(2): 314-317. DOI: 10.3969/j.issn.1001-5256.2021.02.014. |
| [3] |
LOHMANN V, KÖRNER F, KOCH J, et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line[J]. Science, 1999, 285(5424): 110-113. DOI: 10.1126/science.285.5424.110. |
| [4] |
|
| [5] |
PAWLOTSKY JM. Retreatment of hepatitis C virus-infected patients with direct-acting antiviral failures[J]. Semin Liver Dis, 2019, 39(3): 354-368. DOI: 10.1055/s-0039-1687823. |
| [6] |
WEI L, LIM SG, XIE Q, et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: A single-arm, open-label, phase 3 trial[J]. Lancet Gastroenterol Hepatol, 2019, 4(2): 127-134. DOI: 10.1016/S2468-1253(18)30343-1. |
| [7] |
DIETZ J, SUSSER S, VERMEHREN J, et al. Patterns of resistance-associated substitutions in patients with chronic HCV infection following treatment with direct-acting antivirals[J]. Gastroenterology, 2018, 154(4): 976-988. e4. DOI: 10.1053/j.gastro.2017.11.007. |
| [8] |
BAUMERT TF, BERG T, LIM JK, et al. Status of direct-acting antiviral therapy for hepatitis C virus infection and remaining challenges[J]. Gastroenterology, 2019, 156(2): 431-445. DOI: 10.1053/j.gastro.2018.10.024. |
| [9] |
KOMATSU TE, BOYD S, SHERWAT A, et al. Regulatory analysis of effects of hepatitis C Virus NS5A polymorphisms on efficacy of elbasvir and grazoprevir[J]. Gastroenterology, 2017, 152(3): 586-597. DOI: 10.1053/j.gastro.2016.10.017. |
| [10] |
DONALDSON EF, HARRINGTON PR, O'REAR JJ, et al. Clinical evidence and bioinformatics characterization of potential hepatitis C virus resistance pathways for sofosbuvir[J]. Hepatology, 2015, 61(1): 56-65. DOI: 10.1002/hep.27375. |
| [11] |
FELD JJ, JACOBSON IM, HÉZODE C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection[J]. N Engl J Med, 2015, 373(27): 2599-2607. DOI: 10.1056/NEJMoa1512610. |
| [12] |
SORBO MC, CENTO V, DI MAIO VC, et al. Hepatitis C virus drug resistance associated substitutions and their clinical relevance: Update 2018[J]. Drug Resist Updat, 2018, 37: 17-39. DOI: 10.1016/j.drup.2018.01.004. |
| [13] |
ZHOU N, HERNANDEZ D, UELAND J, et al. NS5A sequence heterogeneity and mechanisms of daclatasvir resistance in hepatitis C virus genotype 4 infection[J]. J Infect Dis, 2016, 213(2): 206-215. DOI: 10.1093/infdis/jiv379. |
| [14] |
LAWITZ EJ, DVORY-SOBOL H, DOEHLE BP, et al. Clinical resistance to velpatasvir (GS-5816), a novel pan-genotypic inhibitor of the hepatitis C virus NS5A protein[J]. Antimicrob Agents Chemother, 2016, 60(9): 5368-5378. DOI: 10.1128/AAC.00763-16. |
| [15] |
LIU R, CURRY S, MCMONAGLE P, et al. Susceptibilities of genotype 1a, 1b, and 3 hepatitis C virus variants to the NS5A inhibitor elbasvir[J]. Antimicrob Agents Chemother, 2015, 59(11): 6922-6929. DOI: 10.1128/AAC.01390-15. |



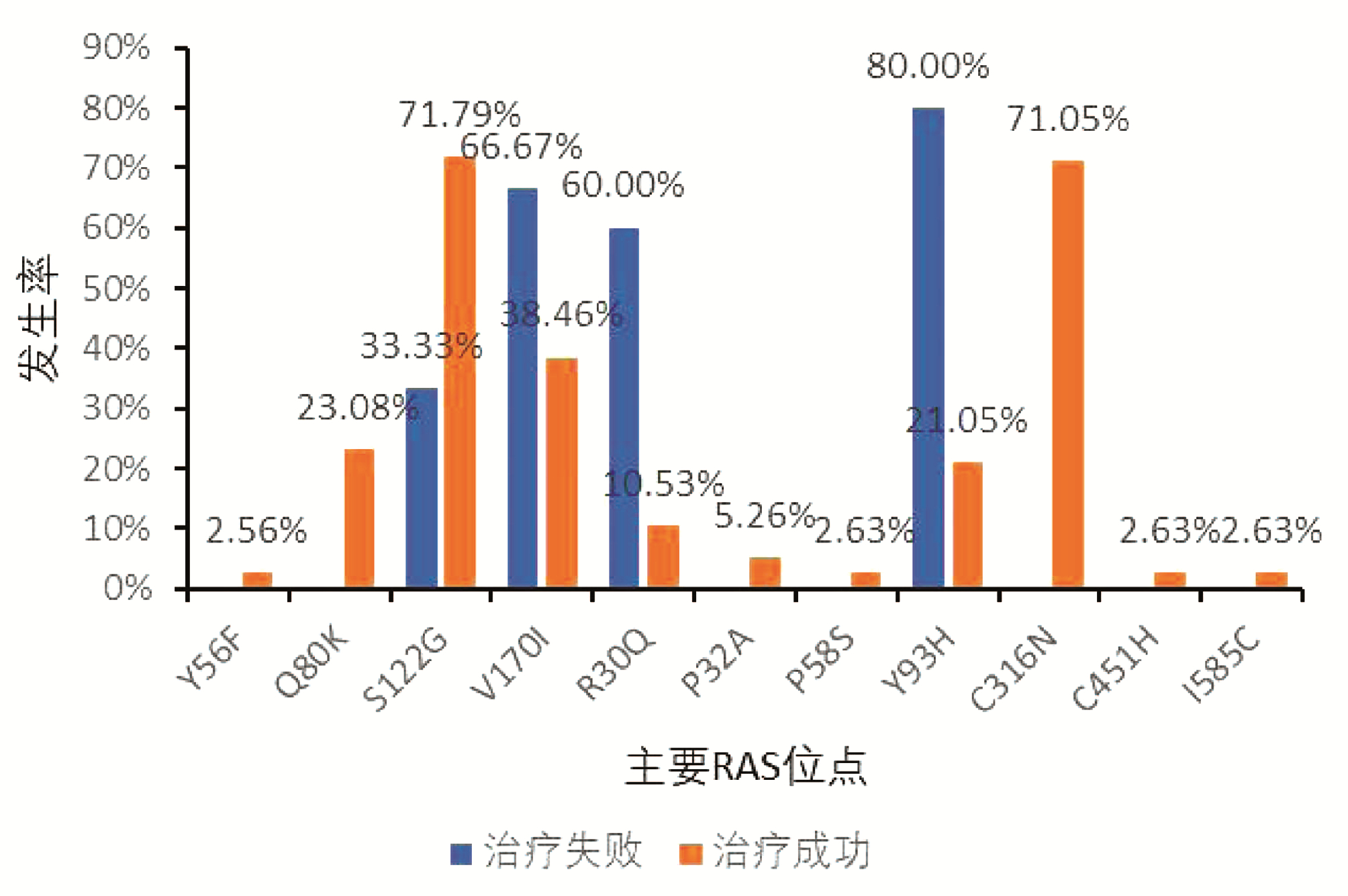




 DownLoad:
DownLoad: