| [1] |
YOUNOSSI ZM, KOENIG AB, ABDELATIF D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes[J]. Hepatology, 2016, 64(1): 73-84. DOI: 10.1002/hep.28431. |
| [2] |
CHOLANKERIL G, PERUMPAIL RB, PHAM EA, et al. Nonalcoholic fatty liver disease: Epidemiology, natural history, and diagnostic challenges[J]. Hepatology, 2016, 64(3): 954. DOI: 10.1002/hep.28719. |
| [3] |
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease[J]. J Hepatol, 2016, 64(6): 1388-1402. DOI: 10.1016/j.jhep.2015.11.004. |
| [4] |
|
| [5] |
SHIHA G, ALSWAT K, AL KHATRY M, et al. Nomenclature and definition of metabolic-associated fatty liver disease: A consensus from the Middle East and north Africa[J]. Lancet Gastroenterol Hepatol, 2021, 6(1): 57-64. DOI: 10.1016/S2468-1253(20)30213-2. |
| [6] |
ESLAM M, GEORGE J. Reply to: Correspondence on "A new definition for metabolic associated fatty liver disease: An international expert consensus statement": MAFLD: Moving from a concept to practice[J]. J Hepatol, 2020, 73(5): 1268-1269. DOI: 10.1016/j.jhep.2020.06.036. |
| [7] |
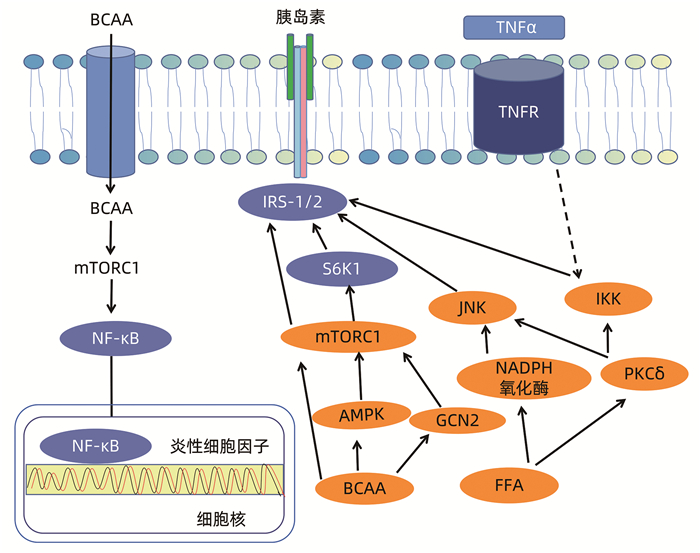
ZHENYUKH O, CIVANTOS E, RUIZ-ORTEGA M, et al. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation[J]. Free Radic Biol Med, 2017, 104: 165-177. DOI: 10.1016/j.freeradbiomed.2017.01.009. |
| [8] |
ZHENYUKH O, GONZÁLEZ-AMOR M, RODRIGUES-DIEZ RR, et al. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation[J]. J Cell Mol Med, 2018, 22(10): 4948-4962. DOI: 10.1111/jcmm.13759. |
| [9] |
SOLON-BIET SM, COGGER VC, PULPITEL T, et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control[J]. Nat Metab, 2019, 1(5): 532-545. DOI: 10.1038/s42255-019-0059-2. |
| [10] |
KIM SJ, TANG T, ABBOTT M, et al. AMPK phosphorylates desnutrin/ATGL and hormone-sensitive lipase to regulate lipolysis and fatty acid oxidation within adipose tissue[J]. Mol Cell Biol, 2016, 36(14): 1961-1976. DOI: 10.1128/MCB.00244-16. |
| [11] |
ZHANG F, ZHAO S, YAN W, et al. Branched chain amino acids cause liver injury in obese/diabetic mice by promoting adipocyte lipolysis and inhibiting hepatic autophagy[J]. EBioMedicine, 2016, 13: 157-167. DOI: 10.1016/j.ebiom.2016.10.013. |
| [12] |
GALARREGUI C, CANTERO I, MARIN-ALEJANDRE BA, et al. Dietary intake of specific amino acids and liver status in subjects with nonalcoholic fatty liver disease: Fatty liver in obesity (FLiO) study[J]. Eur J Nutr, 2021, 60(4): 1769-1780. DOI: 10.1007/s00394-020-02370-6. |
| [13] |
TANAKA H, FUKAHORI S, BABA S, et al. Branched-chain amino acid-rich supplements containing microelements have antioxidant effects on nonalcoholic steatohepatitis in mice[J]. JPEN J Parenter Enteral Nutr, 2016, 40(4): 519-528. DOI: 10.1177/0148607114555160. |
| [14] |
de PASQUALE V, CATERINO M, COSTANZO M, et al. Targeted metabolomic analysis of a mucopolysaccharidosis IIIB mouse model reveals an imbalance of branched-chain amino acid and fatty acid metabolism[J]. Int J Mol Sci, 2020, 21(12): 4211. DOI: 10.3390/ijms21124211. |
| [15] |
YAN X, SUN Q, JI J, et al. Reconstitution of leucine-mediated autophagy via the mTORC1-Barkor pathway in vitro[J]. Autophagy, 2012, 8(2): 213-221. DOI: 10.4161/auto.8.2.18563. |
| [16] |
SON SM, PARK SJ, LEE H, et al. Leucine signals to mTORC1 via its metabolite Acetyl-coenzyme A[J]. Cell Metab, 2019, 29(1): 192-201.e7. DOI: 10.1016/j.cmet.2018.08.013. |
| [17] |
ZHENG R, HUANG S, ZHU J, et al. Leucine attenuates muscle atrophy and autophagosome formation by activating PI3K/AKT/mTOR signaling pathway in rotator cuff tears[J]. Cell Tissue Res, 2019, 378(1): 113-125. DOI: 10.1007/s00441-019-03021-x. |
| [18] |
WU H, DRIDI S, HUANG Y, et al. Leucine decreases intramyocellular lipid deposition in an mTORC1-independent manner in palmitate-treated C2C12 myotubes[J]. Am J Physiol Endocrinol Metab, 2020, 318(2): E152-E163. DOI: 10.1152/ajpendo.00241.2019. |
| [19] |
PENG KY, WATT MJ, RENSEN S, et al. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression[J]. J Lipid Res, 2018, 59(10): 1977-1986. DOI: 10.1194/jlr.M085613. |
| [20] |
VOS M, GEENS A, BÖHM C, et al. Cardiolipin promotes electron transport between ubiquinone and complex I to rescue PINK1 deficiency[J]. J Cell Biol, 2017, 216(3): 695-708. DOI: 10.1083/jcb.201511044. |
| [21] |
PÉREZ-CARRERAS M, DEL HOYO P, MARTÍN MA, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis[J]. Hepatology, 2003, 38(4): 999-1007. DOI: 10.1053/jhep.2003.50398. |
| [22] |
PATTERSON RE, KALAVALAPALLI S, WILLIAMS CM, et al. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity[J]. Am J Physiol Endocrinol Metab, 2016, 310(7): E484-E494. DOI: 10.1152/ajpendo.00492.2015. |
| [23] |
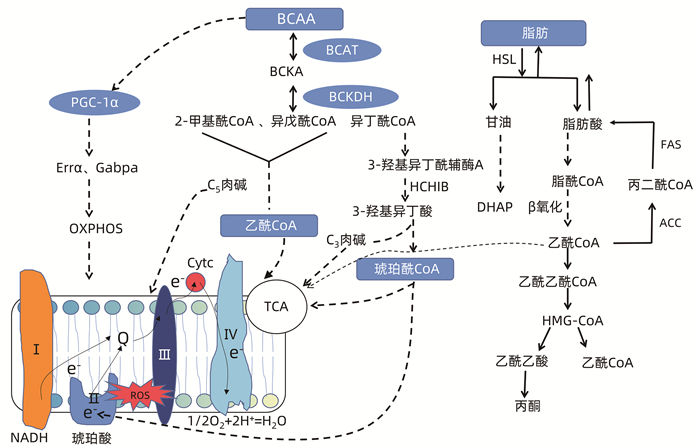
WANG J, LIU Y, LIAN K, et al. BCAA catabolic defect alters glucose metabolism in lean mice[J]. Front Physiol, 2019, 10: 1140. DOI: 10.3389/fphys.2019.01140. |
| [24] |
CHENG S, WIKLUND P, AUTIO R, et al. Adipose tissue dysfunction and altered systemic amino acid metabolism are associated with non-alcoholic fatty liver disease[J]. PLoS One, 2015, 10(10): e0138889. DOI: 10.1371/journal.pone.0138889. |
| [25] |
MUYYARIKKANDY MS, MCLEOD M, MAGUIRE M, et al. Branched chain amino acids and carbohydrate restriction exacerbate ketogenesis and hepatic mitochondrial oxidative dysfunction during NAFLD[J]. FASEB J, 2020, 34(11): 14832-14849. DOI: 10.1096/fj.202001495R. |
| [26] |
GAGGINI M, CARLI F, ROSSO C, et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance[J]. Hepatology, 2018, 67(1): 145-158. DOI: 10.1002/hep.29465. |
| [27] |
XIAO F, HUANG Z, LI H, et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways[J]. Diabetes, 2011, 60(3): 746-756. DOI: 10.2337/db10-1246. |
| [28] |
BUSH NC, BASU R, RIZZA RA, et al. Insulin-mediated FFA suppression is associated with triglyceridemia and insulin sensitivity independent of adiposity[J]. J Clin Endocrinol Metab, 2012, 97(11): 4130-4138. DOI: 10.1210/jc.2012-2285. |
| [29] |
PEREIRA S, PARK E, MORI Y, et al. FFA-induced hepatic insulin resistance in vivo is mediated by PKCδ, NADPH oxidase, and oxidative stress[J]. Am J Physiol Endocrinol Metab, 2014, 307(1): E34-E46. DOI: 10.1152/ajpendo.00436.2013. |
| [30] |
BISWAS D, DAO KT, MERCER A, et al. Branched-chain ketoacid overload inhibits insulin action in the muscle[J]. J Biol Chem, 2020, 295(46): 15597-15621. DOI: 10.1074/jbc.RA120.013121. |
| [31] |
SOLON-BIET SM, COGGER VC, PULPITEL T, et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control[J]. Nat Metab, 2019, 1(5): 532-545. DOI: 10.1038/s42255-019-0059-2. |
| [32] |
HONG SH, LEE KS, KWAK SJ, et al. Minibrain/Dyrk1a regulates food intake through the Sir2-FOXO-sNPF/NPY pathway in drosophila and mammals[J]. PLoS Genet, 2012, 8(8): e1002857. DOI: 10.1371/journal.pgen.1002857. |







 DownLoad:
DownLoad: