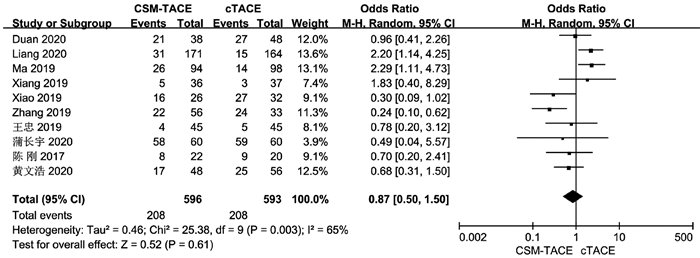
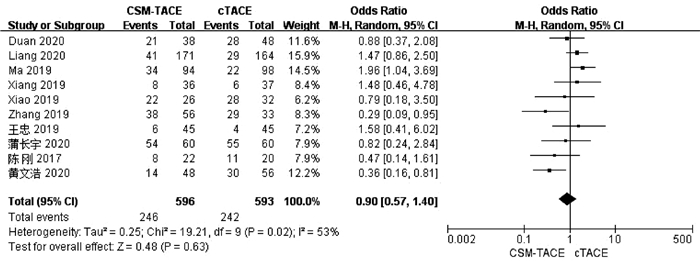
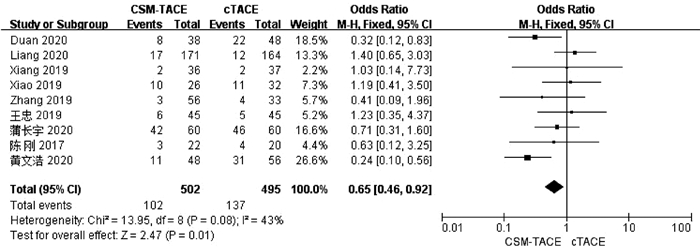
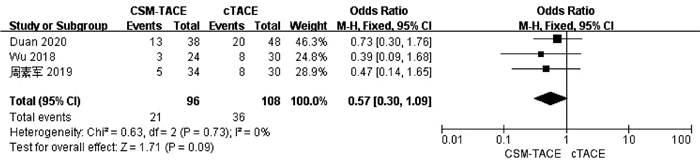
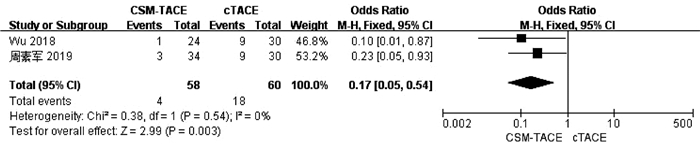
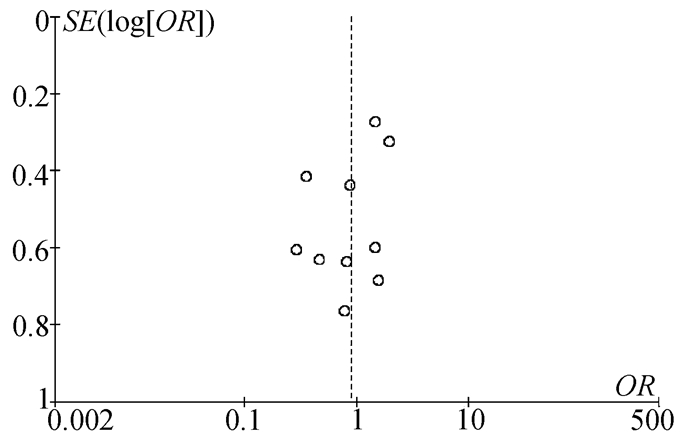
| [1] |
SINGAL AG, LAMPERTICO P, NAHON P. Epidemiology and surveillance for hepatocellular carcinoma: New trends[J]. J Hepatol, 2020, 72(2): 250-261. DOI: 10.1016/j.jhep.2019.08.025. |
| [2] |
ZHOU M, WANG H, ZENG X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2019, 394(10204): 1145-1158. DOI: 10.1016/S0140-6736(19)30427-1. |
| [3] |
Chinese College of Interventionalists, Chinese Medical Doctor Association. Chinese clinical practice guidelines for transarterial chemoembolization of hepatocellular carcinoma[J]. J Intervent Radiol, 2018, 27(12): 1117-1126. DOI: 10.3969/j.issn.1008-794X.2018.12.001. |
| [4] |
de BAERE T, ARAI Y, LENCIONI R, et al. Treatment of liver tumors with lipiodol TACE: Technical recommendations from experts opinion[J]. Cardiovasc Intervent Radiol, 2016, 39(3): 334-343. DOI: 10.1007/s00270-015-1208-y. |
| [5] |
PESAPANE F, NEZAMI N, PATELLA F, et al. New concepts in embolotherapy of HCC[J]. Med Oncol, 2017, 34(4): 58. DOI: 10.1007/s12032-017-0917-2. |
| [6] |
LEE EW, KHAN S. Recent advances in transarterial embolotherapies in the treatment of hepatocellular carcinoma[J]. Clin Mol Hepatol, 2017, 23(4): 265-272. DOI: 10.3350/cmh.2017.0111. |
| [7] |
NAMUR J, CITRON SJ, SELLERS MT, et al. Embolization of hepatocellular carcinoma with drug-eluting beads: Doxorubicin tissue concentration and distribution in patient liver explants[J]. J Hepatol, 2011, 55(6): 1332-1338. DOI: 10.1016/j.jhep.2011.03.024. |
| [8] |
ZHOU GH, HAN J, SUN JH, et al. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheres Ⓡ beads in Chinese hepatocellular carcinoma patients[J]. BMC Cancer, 2018, 18(1): 644. DOI: 10.1186/s12885-018-4566-4. |
| [9] |
WU B, ZHOU J, LING G, et al. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: A short-term efficacy and safety study[J]. World J Surg Oncol, 2018, 16(1): 69. DOI: 10.1186/s12957-018-1368-8. |
| [10] |
HUANG WH, FENG GS. Clinical analysis of polyvinyl alcohol callispheres in interventional embolization therapy for primary hepatocellular carcinoma[J]. J Pract Oncol, 2020, 35(3): 260-264. DOI: 10.13267/j.cnki.syzlzz.2020.03.014. |
| [11] |
PARMAR MK, TORRI V, STEWART L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints[J]. Stat Med, 1998, 17(24): 2815-2834. DOI: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8.
|
| [12] |
JADAD AR, MOORE RA, CARROLL D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?[J]. Control Clin Trials, 1996, 17(1): 1-12. DOI: 10.1016/0197-2456(95)00134-4. |
| [13] |
MA Y, ZHAO C, ZHAO H, et al. Comparison of treatment efficacy and safety between drug-eluting bead transarterial chemoembolization with CalliSpheresⓇ microspheres and conventional transarterial chemoembolization as first-line treatment in hepatocellular carcinoma patients[J]. Am J Transl Res, 2019, 11(12): 7456-7470.
|
| [14] |
ZHANG ZS, LI HZ, MA C, et al. Conventional versus drug-eluting beads chemoembolization for infiltrative hepatocellular carcinoma: A comparison of efficacy and safety[J]. BMC Cancer, 2019, 19(1): 1162. DOI: 10.1186/s12885-019-6386-6. |
| [15] |
XIAO YD, MA C, ZHANG ZS, et al. Safety and efficacy assessment of transarterial chemoembolization using drug-eluting beads in patients with hepatocellular carcinoma and arterioportal shunt: A single-center experience[J]. Cancer Manag Res, 2019, 11: 1551-1557. DOI: 10.2147/CMAR.S193948. |
| [16] |
XIANG H, LONG L, YAO Y, et al. CalliSpheres drug-eluting bead transcatheter arterial chemoembolization presents with better efficacy and equal safety compared to conventional TACE in treating patients with hepatocellular carcinoma[J]. Technol Cancer Res Treat, 2019, 18: 1533033819830751. DOI: 10.1177/1533033819830751. |
| [17] |
LI H, WU F, DUAN M, et al. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: A comparison of efficacy and safety[J]. Medicine (Baltimore), 2019, 98(21): e15314. DOI: 10.1097/MD.0000000000015314. |
| [18] |
DUAN XH, JU SG, HAN XW, et al. Arsenic trioxide-eluting Callispheres beads is more effective and equally tolerant compared with arsenic trioxide/lipiodol emulsion in the transcatheter arterial chemoembolization treatment for unresectable hepatocellular carcinoma patients[J]. Eur Rev Med Pharmacol Sci, 2020, 24(3): 1468-1480. DOI: 10.26355/eurrev_202002_20206. |
| [19] |
LIANG B, XIANG H, MA C, et al. Comparison of chemoembolization with CalliSpheres(Ⓡ) microspheres and conventional chemoembolization in the treatment of hepatocellular carcinoma: A multicenter retrospective study[J]. Cancer Manag Res, 2020, 12: 941-956. DOI: 10.2147/CMAR.S187203. |
| [20] |
ZHOU SJ, WU BL, ZHOU J, et al. Short-term efficacy and safety of drug-eluting beads transarterial chemoembolization in the treatment of advanced primary hepatocellular carcinoma[J]. Med J Wuhan Univ, 2019, 40(5): 742-746. DOI: 10.14188/j.1671-8852.2018.1170. |
| [21] |
WANG Z, LIU QY, YANG W, et al. Clinical value of transarterial chemoembolization with CalliSpheres drug-eluting beads manufactured in China in treatment of unresectable liver cancer[J]. Chin Hepatol, 2019, 24(7): 767-770. DOI: 10.3969/j.issn.1008-1704.2019.07.014. |
| [22] |
PU CY, CHEN Q. Application of drug-eluting beads in transarterial chemoembolization for advanced liver cancer and its effect on immune function[J]. Chin Hepatol, 2020, 25(1): 42-46. DOI: 10.3969/j.issn.1008-1704.2020.01.015. |
| [23] |
LIU JF, DUAN XH, REN JZ, et al. Comparative effect of CalliSpheres drug loading microspheres and lipiodol transarterial chemoembolization in the treatment of huge primary liver cancer[J]. Chin J Hepatol, 2019, 27(6): 460-462. DOI: 10.3760/cma.j.issn.1007-3418.2019.06.014. |
| [24] |
LI FY, SUN YM, SHI MB. Value of drug-eluting beads versus ultra-fluid lipiodol in transarterial chemoembolization for liver cancer[J]. Mod Digest Interv, 2020, 25(5): 649-652. DOI: 10.3969/j.issn.1672-2159.2020.05.022. |
| [25] |
CHEN G, ZHANG D, YING YC, et al. Clinical investigation on transarterial chemoembolization with indigenous drug-eluting beads in treatment of unresectable hepatocellular carcinoma[J]. J Zhejiang Univ(Med Sci), 2017, 46(1): 44-51. DOI: 10.3785/j.issn.1008-9292.2017.02.07. |
| [26] |
HA Y, LEE JB, SHIM JH, et al. Validation and reappraisal of the assessment for retreatment with transarterial chemoembolization score for unresectable non-metastatic hepatocellular carcinoma in a hepatitis B virus-endemic region[J]. Eur Radiol, 2016, 26(10): 3510-3518. DOI: 10.1007/s00330-015-4185-2. |
| [27] |
GOLFIERI R, GIAMPALMA E, RENZULLI M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma[J]. Br J Cancer, 2014, 111(2): 255-264. DOI: 10.1038/bjc.2014.199. |
| [28] |
MEMON K, KULIK L, LEWANDOWSKI RJ, et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times[J]. Gastroenterology, 2011, 141(2): 526-535. DOI: 10.1053/j.gastro.2011.04.054. |
| [29] |
YIN WL, LIAN J, XIAO SX, et al. Clinical effect of drug-eluting beads and conventional transcatheter arterial chemoembolization in treatment of unre-sectable liver cancer: A meta-analysis[J]. J Clin Hepatol, 2019, 35(6): 1270-1275. DOI: 10.3969/j.issn.1001-5256.2019.06.018. |
| [30] |
CHEN P, YUAN P, CHEN B, et al. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: A systematic review and meta-analysis[J]. Clin Res Hepatol Gastroenterol, 2017, 41(1): 75-85. DOI: 10.1016/j.clinre.2016.05.013. |
| [31] |
JIANG S, LI GJ, ZHOU ZQ, et al. Interventional chemoembolization with CalliSpheres-loaded microspheres for the treatment of advanced hepatocellular carcinoma[J/CD]. Chin J Inter Rad(Electronic Edition), 2017, 5(3): 174-178. DOI: 10.3877/cma.j.issn.2095-5782.2017.03.013. |
| [32] |
XIE XC, ZHAO W, FAN HJ, et al. Safety and efficacy analysis of CalliSpheres drug-eluting beads TACE in the treatment of advanced liver cancer[J]. J Pract Radiol, 2020, 36(5): 792-795. DOI: 10.3969/j.issn.1002-1671.2020.05.026. |
| [33] |
HONG K, KHWAJA A, LIAPI E, et al. New intra-arterial drug delivery system for the treatment of liver cancer: Preclinical assessment in a rabbit model of liver cancer[J]. Clin Cancer Res, 2006, 12(8): 2563-2567. DOI: 10.1158/1078-0432.CCR-05-2225. |
| [34] |
TAYLOR RR, TANG Y, GONZALEZ MV, et al. Irinotecan drug eluting beads for use in chemoembolization: In vitro and in vivo evaluation of drug release properties[J]. Eur J Pharm Sci, 2007, 30(1): 7-14. DOI: 10.1016/j.ejps.2006.09.002. |
| [35] |
LAMMER J, MALAGARI K, VOGL T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study[J]. Cardiovasc Intervent Radiol, 2010, 33(1): 41-52. DOI: 10.1007/s00270-009-9711-7. |



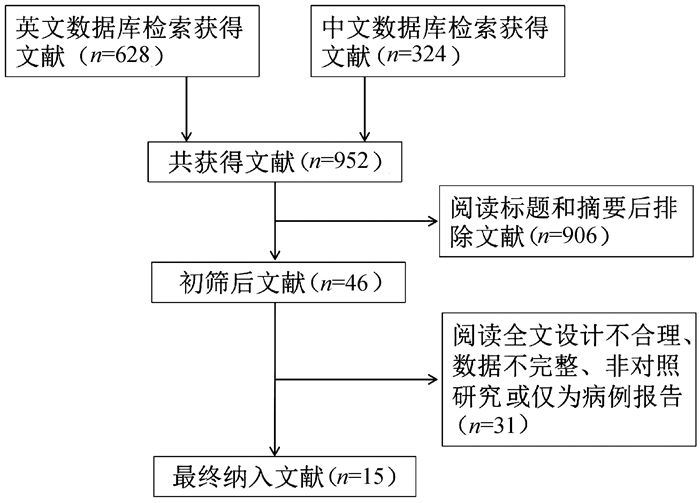




 DownLoad:
DownLoad: