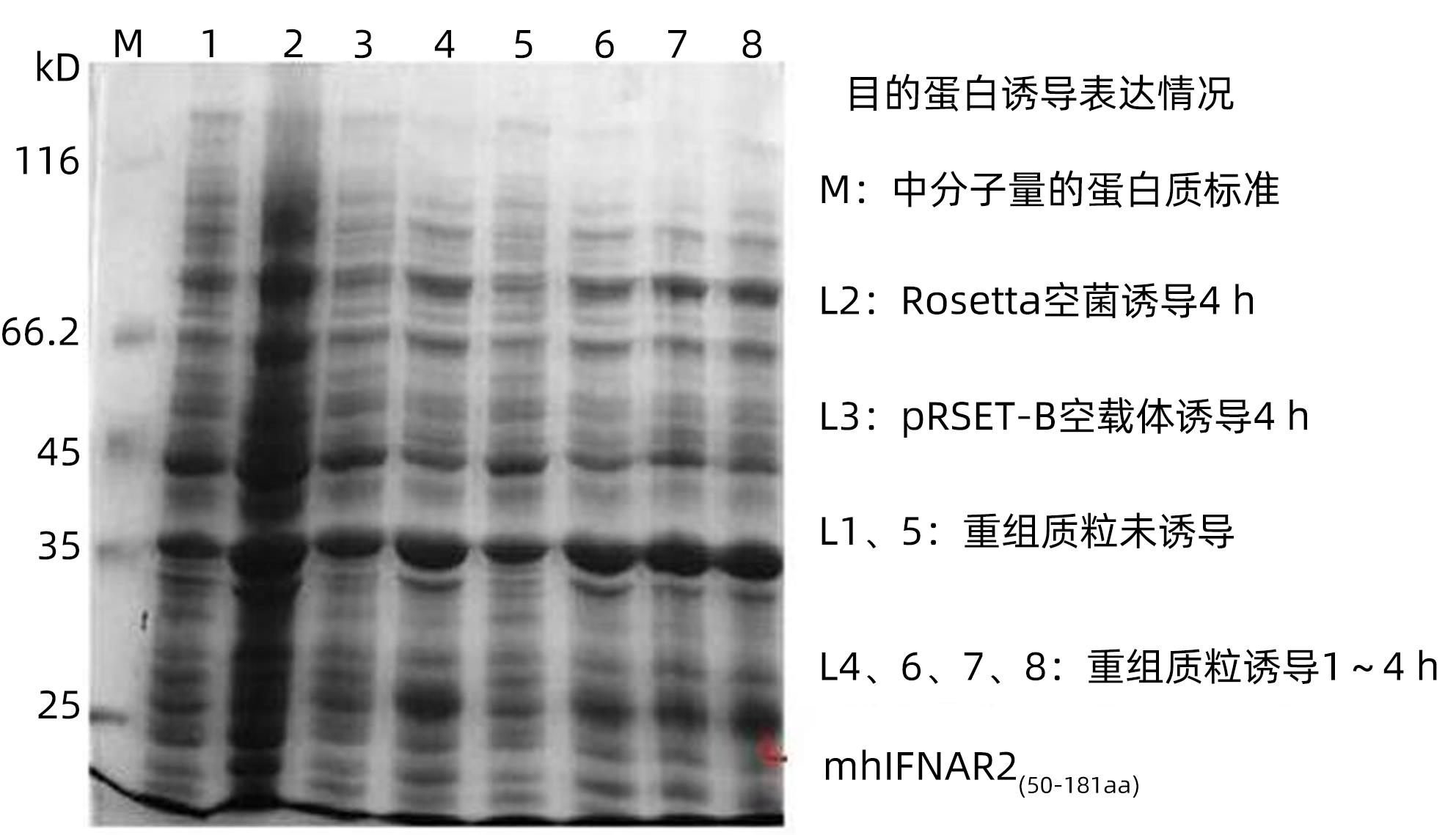
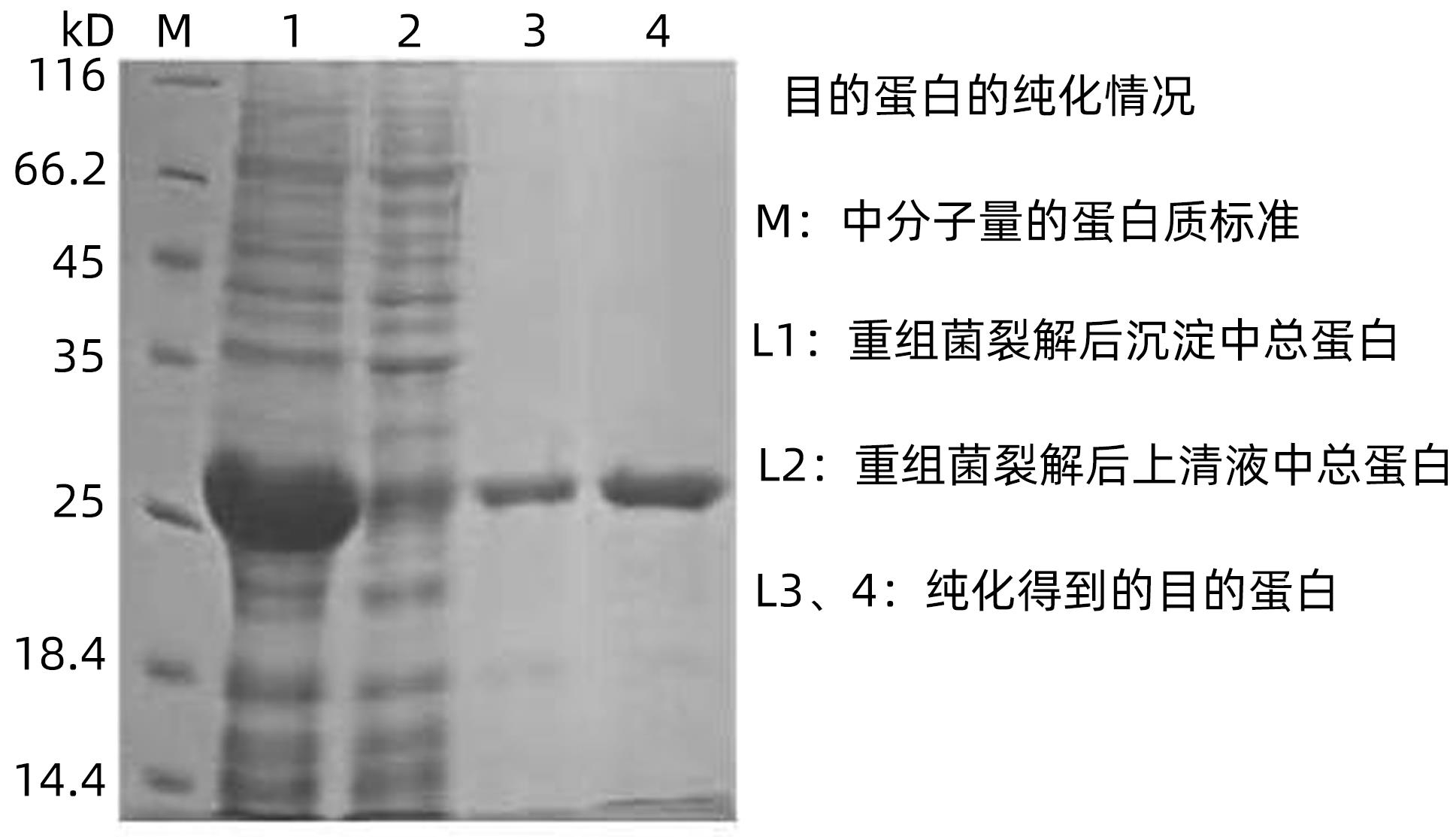
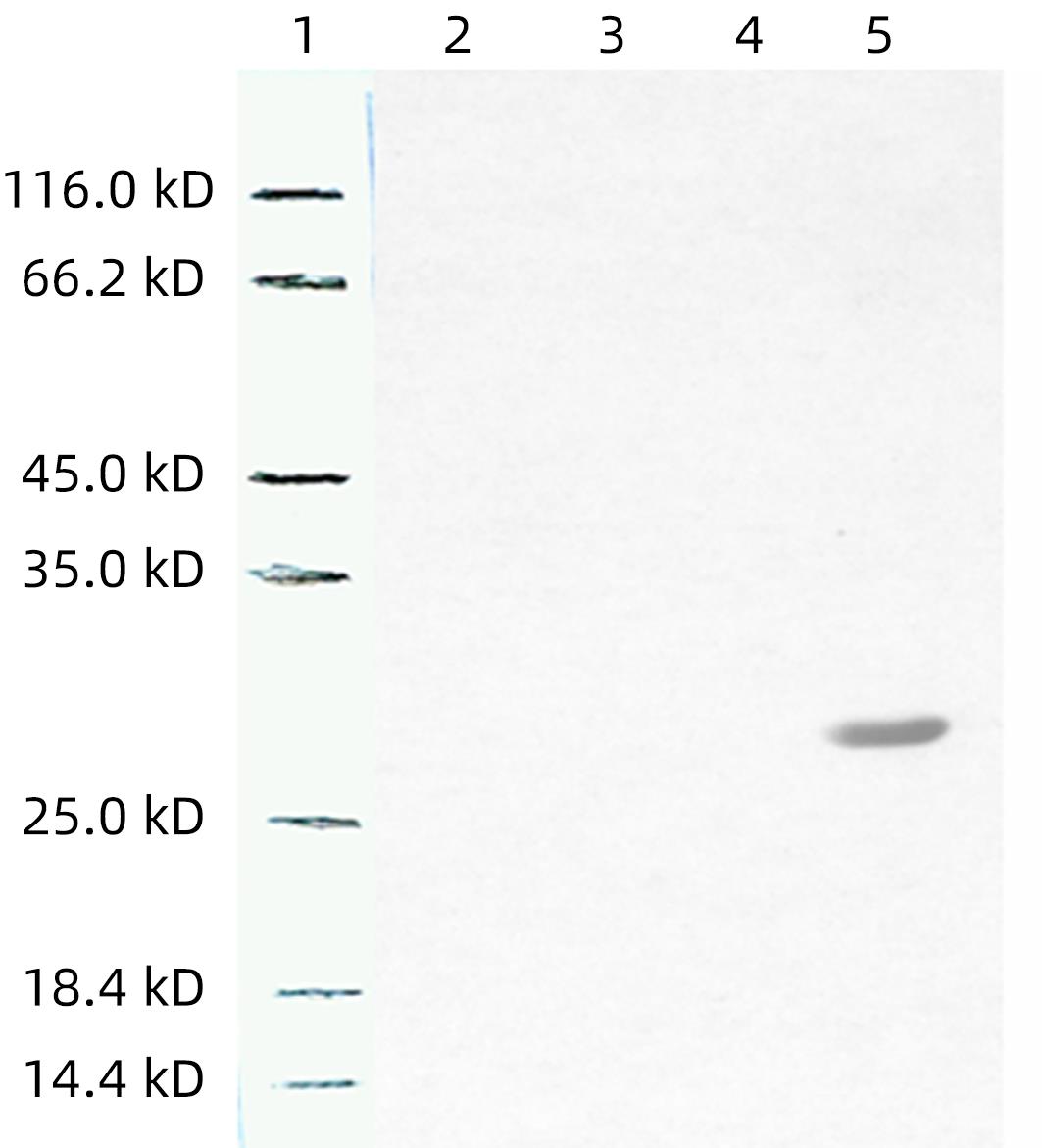
| [1] |
ERSVAER E, SKAVLAND J, ULVESTAD E, et al. Effects of interferon gamma on native human acute myelogenous leukaemia cells[J]. Cancer Immunol Immunother, 2007, 56( 1): 13- 24. DOI: 10.1007/s00262-006-0159-1. |
| [2] |
MERLI P, QUINTARELLI C, STROCCHIO L, et al. The role of interferon-gamma and its signaling pathway in pediatric hematological disorders[J]. Pediatr Blood Cancer, 2021, 68( 4): e28900. DOI: 10.1002/pbc.28900. |
| [3] |
SCHRODER K, HERTZOG PJ, RAVASI T, et al. Interferon-gamma: An overview of signals, mechanisms and functions[J]. J Leukoc Biol, 2004, 75( 2): 163- 189. DOI: 10.1189/jlb.0603252. |
| [4] |
BHAT MY, SOLANKI HS, ADVANI J, et al. Comprehensive network map of interferon gamma signaling[J]. J Cell Commun Signal, 2018, 12( 4): 745- 751. DOI: 10.1007/s12079-018-0486-y. |
| [5] |
WANG BJ, TIAN YJ, MENG ZJ, et al. Establishing a new animal model for hepadnaviral infection: Susceptibility of Chinese Marmota-species to woodchuck hepatitis virus infection[J]. J Gen Virol, 2011, 92( Pt 3): 681- 691. DOI: 10.1099/vir.0.025023-0. |
| [6] |
|
| [7] |
FAN HB, ZHU ZN, WANG Y, et al. Molecular characterization of the type I IFN receptor in two woodchuck species and detection of its expression in liver samples from woodchucks infected with woodchuck hepatitis virus(WHV)[J]. Cytokine, 2012, 60( 1): 179- 185. DOI: 10.1016/j.cyto.2012.05.013.2012. |
| [8] |
SCHREIBER G. The role of type I interferons in the pathogenesis and treatment of COVID-19[J]. Front Immunol, 2020, 11: 595739. DOI: 10.3389/fimmu.2020.595739. |
| [9] |
MCNAB F, MAYER-BARBER K, SHER A, et al. Type I interferons in infectious disease[J]. Nat Rev Immunol, 2015, 15( 2): 87- 103. DOI: 10.1038/nri3787. |
| [10] |
MURIRA A, LAMARRE A. Type-I interferon responses: From friend to foe in the battle against chronic viral infection[J]. Front Immunol, 2016, 7: 609. DOI: 10.3389/fimmu.2016.00609. |
| [11] |
BASTARD P, MANRY J, CHEN J, et al. Herpes simplex encephalitis in a patient with a distinctive form of inherited IFNAR1 deficiency[J]. J Clin Invest, 2021, 131( 1): e139980. DOI: 10.1172/JCI139980. |
| [12] |
KHANMOHAMMADI S, REZAEI N, KHAZAEI M, et al. A case of autosomal recessive interferon alpha/beta receptor alpha chain(IFNAR1) deficiency with severe COVID-19[J]. J Clin Immunol, 2022, 42( 1): 19- 24. DOI: 10.1007/s10875-021-01166-5. |
| [13] |
PLATANIAS LC. Mechanisms of type-I- and type-II-interferon-mediated signalling[J]. Nat Rev Immunol, 2005, 5( 5): 375- 386. DOI: 10.1038/nri1604. |
| [14] |
|
| [15] |
|
| [16] |
LU YP, XU Y, YANG DL, et al. Molecular characterization of woodchuck type I interferons and their expression by woodchuck peripheral blood lymphocytes[J]. Cytokine, 2008, 41( 2): 127- 135. DOI: 10.1016/j.cyto.2007.11.002. |
| [17] |
WANG BJ, LOHRENGEL B, LU YP, et al. Molecular characterization of woodchuck interleukin 15(wIL-15) and detection of its expression in liver samples of woodchucks infected with woodchuck hepatitis virus(WHV)[J]. Cytokine, 2005, 32( 6): 296- 303. DOI: 10.1016/j.cyto.2005.11.007. |
| [18] |
YANG Y, HUANG H, ZHANG ZH, et al. Cloning, expression and polyclonal antibody preparation of the asialoglycoprotein receptor of Marmota Himalayan[J]. J Huazhong Univ Sci Technol(Med Sci), 2007, 27( 4): 411- 414. DOI: 10.1007/s11596-007-0415-4. |
| [19] |
SHEEHAN KC, LAI KS, DUNN GP, et al. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1(IFNAR-1) from mice immunized by in vivo hydrodynamic transfection[J]. J Interferon Cytokine Res, 2006, 26( 11): 804- 819. DOI: 10.1089/jir.2006.26.804. |
| [20] |
BENOIT P, MAGUIRE D, PLAVEC I, et al. A monoclonal antibody to recombinant human IFN-alpha receptor inhibits biologic activity of several species of human IFN-alpha, IFN-beta, and IFN-omega. Detection of heterogeneity of the cellular type I IFN receptor[J]. J Immunol, 1993, 150( 3): 707- 716.
|
| [21] |
COLAMONICI OR, D’ALESSANDRO F, DIAZ MO, et al. Characterization of three monoclonal antibodies that recognize the interferon alpha 2 receptor[J]. Proc Natl Acad Sci U S A, 1990, 87( 18): 7230- 7234. DOI: 10.1073/pnas.87.18.7230. |
| [22] |
UZÉ G, LUTFALLA G, EID P, et al. Murine tumor cells expressing the gene for the human interferon alpha beta receptor elicit antibodies in syngeneic mice to the active form of the receptor[J]. Eur J Immunol, 1991, 21( 2): 447- 451. DOI: 10.1002/eji.1830210229. |








 DownLoad:
DownLoad: