藏红花醛对脂多糖诱导的脓毒症相关肝损伤小鼠模型的作用及其机制
DOI: 10.3969/j.issn.1001-5256.2023.11.019
伦理学声明:本研究方案于2021年11月19日经由西南医科大学实验动物伦理委员会审批,批号:20211119-047,符合实验室动物管理与使用准则。
利益冲突声明:本研究不存在任何利益冲突。
作者贡献声明:陈意负责课题设计,资料分析,撰写论文;陈意、陈羿帆参与动物造模及实验;李童希、白俊杰、杜毅超、谭鹏负责数据分析和细胞实验;付文广负责实验设计,拟定写作思路,指导撰写文章并最后定稿。
Effect and mechanism of safranal in a mouse model of sepsis-related liver injury induced by lipopolysaccharide
-
摘要:
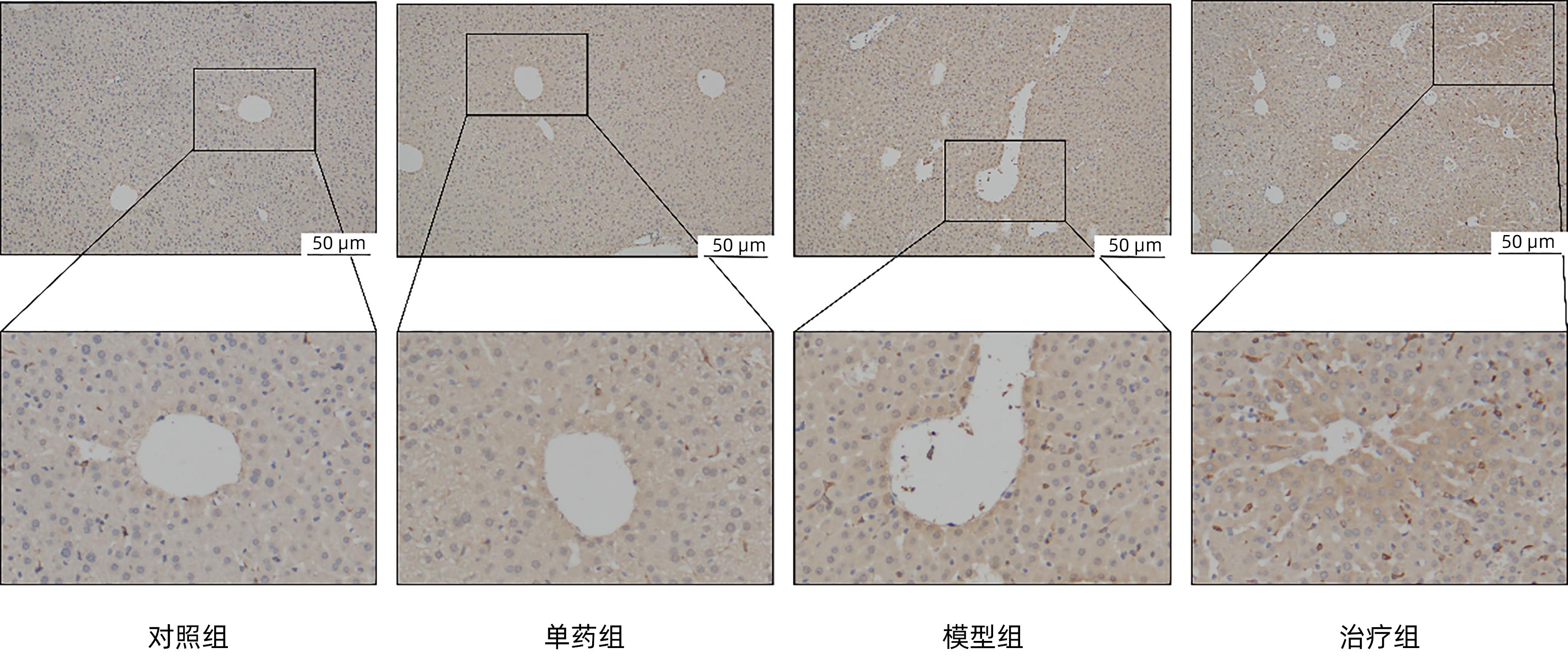
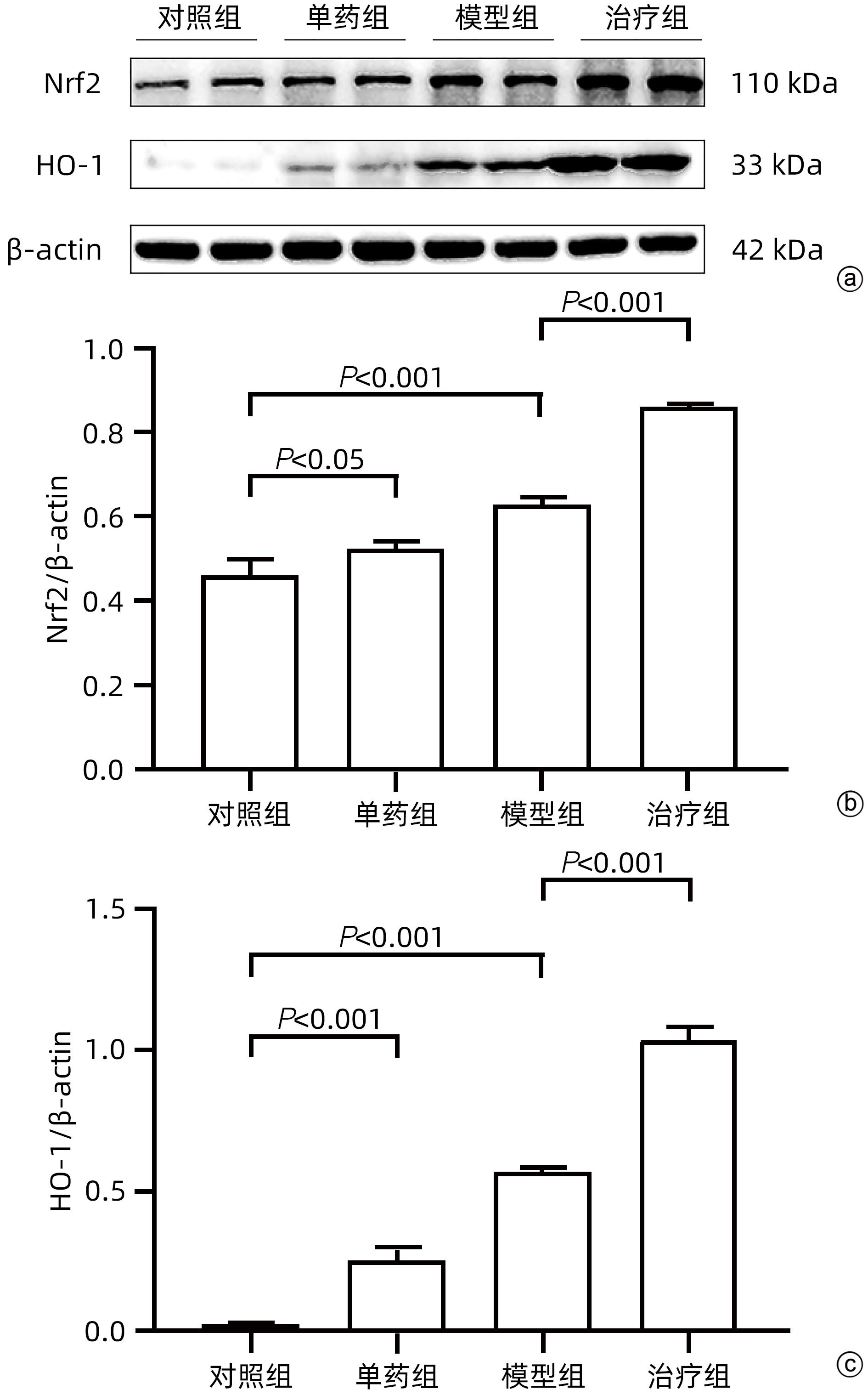
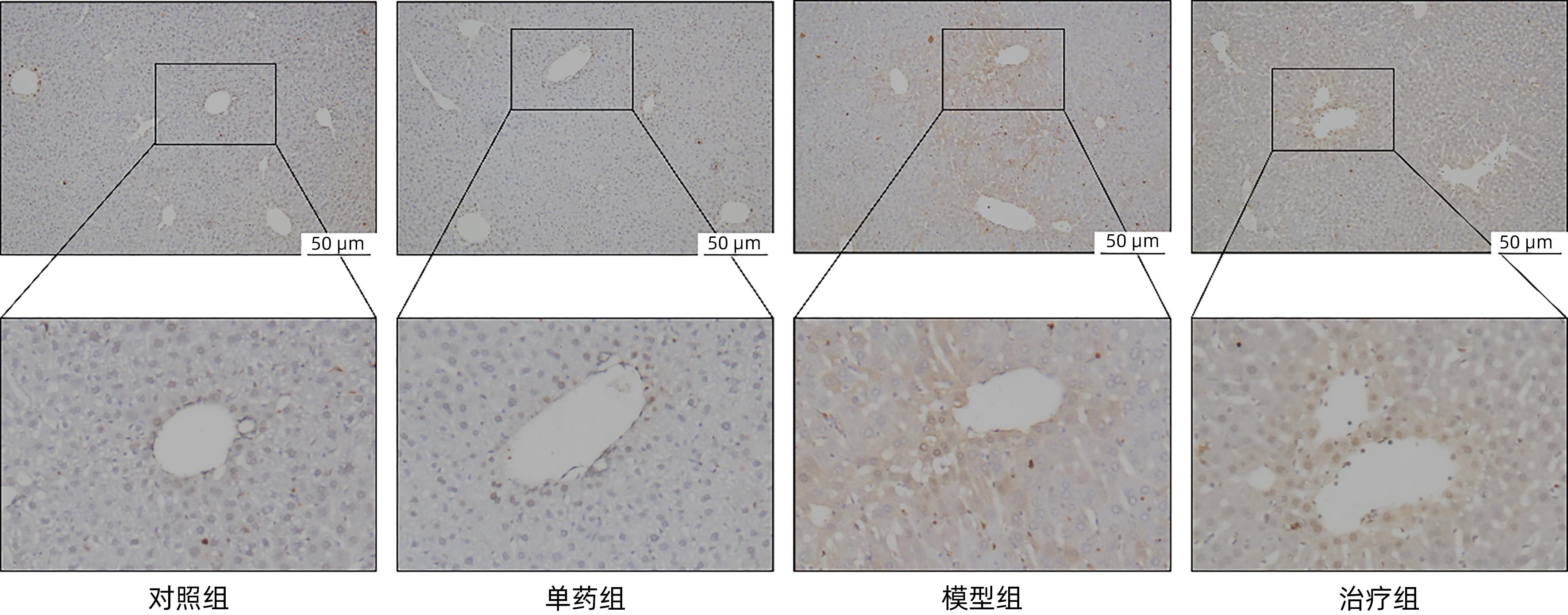
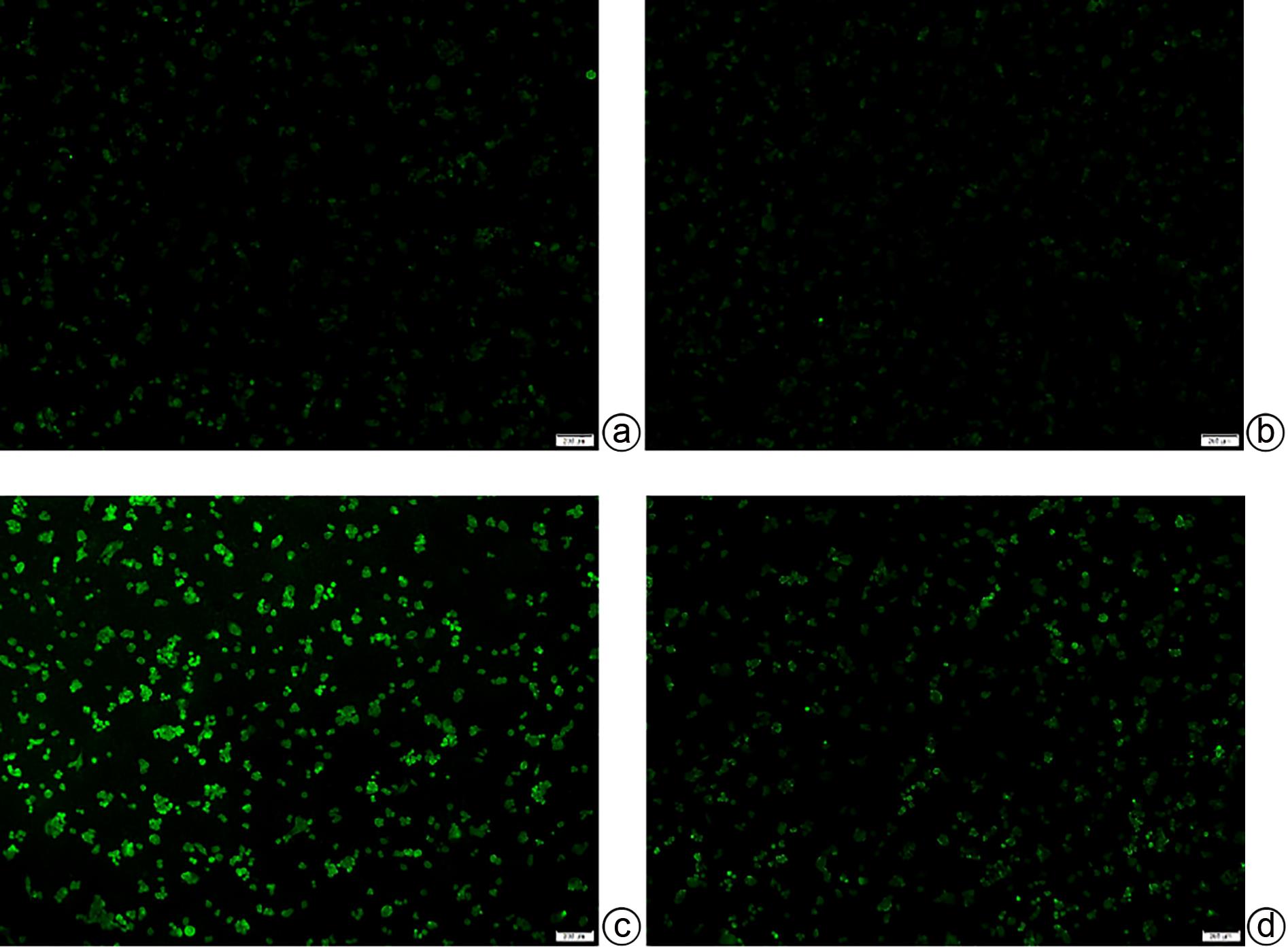
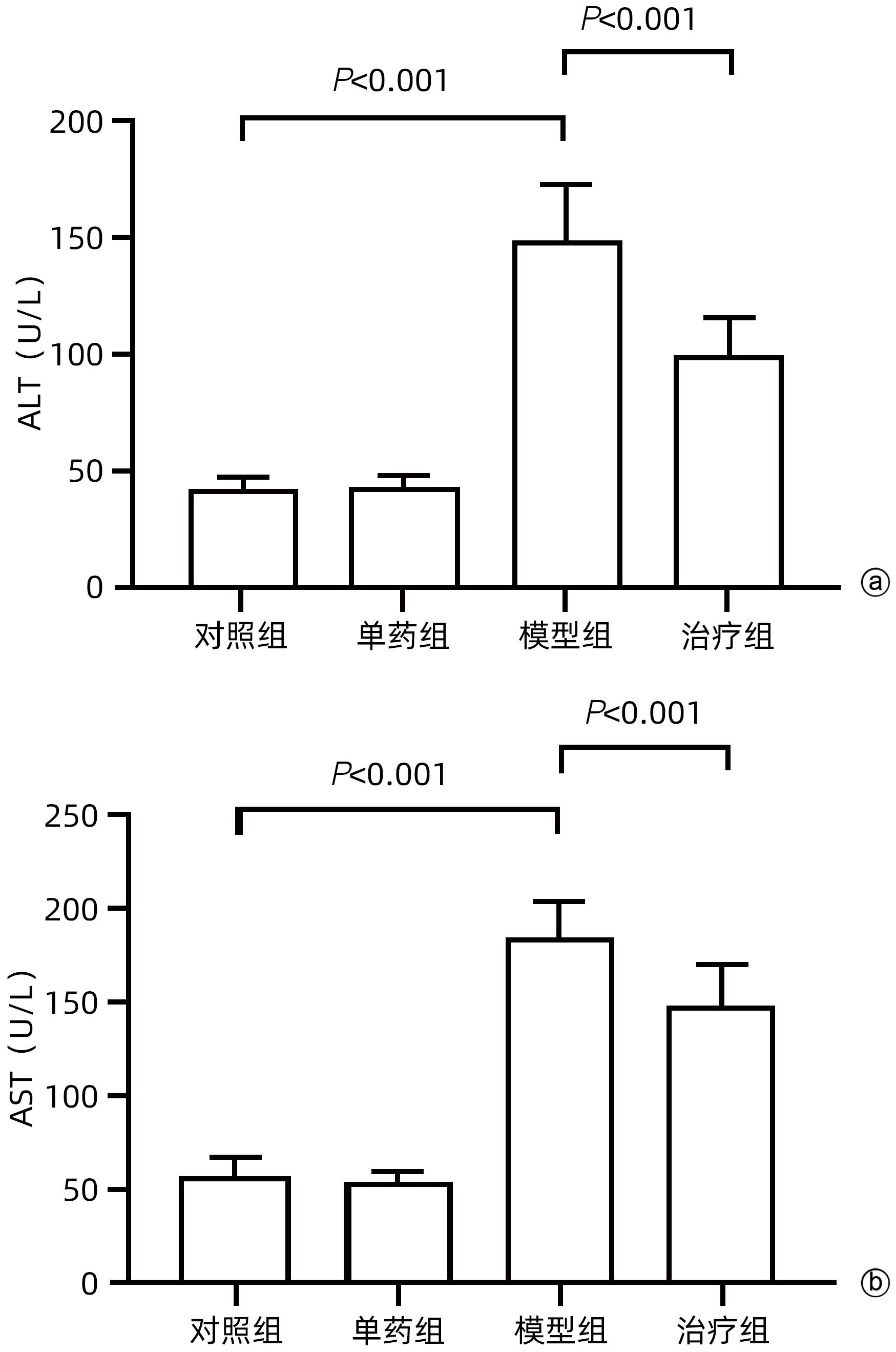
目的 探讨藏红花醛对脂多糖(LPS)诱导的脓毒症相关肝损伤(SRLI)小鼠模型的保护作用及其机制。 方法 采用简单随机分组法将32只实验用C57BL/6雄性小鼠分为对照组、单药组、模型组和治疗组,每组各8只。单药组和治疗组腹腔注射藏红花醛(60 mg/kg)预处理7天,模型组和治疗组腹腔注射LPS(10 mg/kg)诱导急性肝损伤。检测各组小鼠血清ALT、AST活性,HE染色观察肝组织切片标本,免疫组化分析信号通路下游蛋白血红素加氧酶-1(HO-1)的表达差异,TUNEL法分析肝细胞凋亡情况,Western Blot法分析肝组织总蛋白[核因子NF-E2相关因子2(Nrf2)、HO-1]表达差异。采用藏红花醛(100 μmol/L)预处理人类肝细胞系L02细胞,通过LPS 100 ng/mL诱导急性肝细胞损伤,DCFH-DA荧光标记氧自由基。 结果 藏红花醛预处理后,治疗组小鼠ALT、AST明显低于模型组(P值均<0.01),治疗组小鼠肝脏维持了较为完整的假小叶结构且坏死面积更小。治疗组小鼠经藏红花醛+LPS处理后肝组织Nrf2、HO-1表达水平相较于模型组明显升高(P值均<0.001),免疫组化结果显示,藏红花醛预处理增加了HO-1阳性细胞的数量。在LPS诱导的急性肝损伤细胞模型中,治疗组相较于模型组ROS的产生量明显减少。 结论 藏红花醛可通过Nrf-2/HO-1通路介导LPS诱导的小鼠SRLI的保护作用。 -
关键词:
- 脓毒症 /
- 肝损伤 /
- 藏红花醛 /
- NF-E2相关因子2 /
- 血红素加氧酶-1
Abstract:Objective To investigate the protective effect of safranal against sepsis-related liver injury (SRLI) induced by lipopolysaccharide (LPS) in mice and its mechanism. Methods A total of 32 experimental male C57BL/6 mice were divided into control group, single drug group, model group, and treatment group using the simple random method, with 8 mice in each group. The mice in the single drug group and the treatment group were intraperitoneally injected with safranal (60 mg/kg) for 7 days of pretreatment, and the mice in the model group and the treatment group were intraperitoneally injected with LPS (10 mg/kg) to induce acute liver injury. The activities of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured; HE staining was used to observe liver tissue sections; immunohistochemistry was used to analyze the expression of the downstream protein heme oxygenase-1 (HO-1) in the signal pathway; TUNEL was used to analyze the apoptosis of hepatocytes; Western blot was used to measure the expression of total proteins (nuclear factor erythroid 2-related factor 2 [Nrf-2] and HO-1) in liver tissue. The human liver cell line L02 was pretreated with safranal (100 μmol/L), followed by induction of acute hepatocellular injury with LPS (100 ng/mL), and DCFH-DA fluorescent labeling was used to detect reactive oxygen species (ROS). Results After safranal pretreatment, the treatment group had significantly lower levels of ALT and AST than the model group (both P<0.001), with a relatively intact pseudolobular structure and a smaller necrotic area in the liver. Compared with the model group, the treatment group had significant increases in the expression levels of Nrf2 and HO-1 in liver tissue after safranal+LPS treatment (both P<0.001), and immunohistochemistry showed that safranal pretreatment increased the number of HO-1-positive cells. In the cell model of LPS-induced acute liver injury, the treatment group had a significant reduction in the production of ROS compared with the model group. Conclusion Safranal can exert a protective effect against SRLI induced by LPS in mice through the Nrf2/HO-1 pathway. -
Key words:
- Sepsis /
- Liver Injury /
- Safranal /
- NF-E2-Related Factor 2 /
- Heme Oxygenase-1
-
-
[1] LI Q, TAN Y, CHEN SN, et al. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling[J]. J Recept Signal Transduct Res, 2021, 41( 3): 294- 303. DOI: 10.1080/10799893.2020.1808675. [2] ZHANG J, LI XY, CHEN JP, et al. Advances in microRNA and pathogenesis of sepsis[J]. Trauma Crit Care Med, 2021, 9( 4): 315- 318. DOI: 10.16048/j.issn.2095-5561.2021.04.20.张杰, 李小悦, 陈建平, 等. 微小RNA与脓毒症发病机制研究进展[J]. 创伤与急危重病医学, 2021, 9( 4): 315- 318. DOI: 10.16048/j.issn.2095-5561.2021.04.20. [3] CARPINO G, DEL BEN M, PASTORI D, et al. Increased liver localization of lipopolysaccharides in human and experimental NAFLD[J]. Hepatology, 2020, 72( 2): 470- 485. DOI: 10.1002/hep.31056. [4] JING ZT, LIU W, XUE CR, et al. AKT activator SC79 protects hepatocytes from TNF-α-mediated apoptosis and alleviates d-Gal/LPS-induced liver injury[J]. Am J Physiol Gastrointest Liver Physiol, 2019, 316( 3): G387- G396. DOI: 10.1152/ajpgi.00350.2018. [5] YAN CY, OUYANG SH, WANG X, et al. Celastrol ameliorates Propionibacterium acnes/LPS-induced liver damage and MSU-induced gouty arthritis via inhibiting K63 deubiquitination of NLRP3[J]. Phytomedicine, 2021, 80: 153398. DOI: 10.1016/j.phymed.2020.153398. [6] CIESIELSKA A, MATYJEK M, KWIATKOWSKA K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling[J]. Cell Mol Life Sci, 2021, 78( 4): 1233- 1261. DOI: 10.1007/s00018-020-03656-y. [7] ISHIDA K, KAJI K, SATO S, et al. Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway[J]. J Nutr Biochem, 2021, 89: 108573. DOI: 10.1016/j.jnutbio.2020.108573. [8] LIAO Y, HE YH, LUO YW. Role of oxidative stress in acute liver injury[J]. J Clin Hepatol, 2022, 38( 10): 2402- 2407. DOI: 10.3969/j.issn.1001-5256.2022.10.039.廖月, 何毅怀, 罗亚文. 氧化应激在急性肝损伤中的作用[J]. 临床肝胆病杂志, 2022, 38( 10): 2402- 2407. DOI: 10.3969/j.issn.1001-5256.2022.10.039. [9] YANG WC, WANG YX, ZHANG CG, et al. Maresin1 protect against ferroptosis-induced liver injury through ROS inhibition and Nrf2/HO-1/GPX4 activation[J]. Front Pharmacol, 2022, 13: 865689. DOI: 10.3389/fphar.2022.865689. [10] ZHANG HB, YUAN B, HUANG HF, et al. Gastrodin induced HO-1 and Nrf2 up-regulation to alleviate H2O2-induced oxidative stress in mouse liver sinusoidal endothelial cells through p38 MAPK phosphorylation[J]. Braz J Med Biol Res, 2018, 51( 10): e7439. DOI: 10.1590/1414-431x20187439. [11] FOROUZANFAR F, ASADPOUR E, HOSSEINZADEH H, et al. Safranal protects against ischemia-induced PC12 cell injury through inhibiting oxidative stress and apoptosis[J]. Naunyn Schmiedebergs Arch Pharmacol, 2021, 394( 4): 707- 716. DOI: 10.1007/s00210-020-01999-8. [12] HOSSEINI A, RAZAVI BM, HOSSEINZADEH H. Pharmacokinetic properties of saffron and its active components[J]. Eur J Drug Metab Pharmacokinet, 2018, 43( 4): 383- 390. DOI: 10.1007/s13318-017-0449-3. [13] NANDA SJ, MADAN K. The role of Safranal and saffron stigma extracts in oxidative stress, diseases and photoaging: A systematic review[J]. Heliyon, 2021, 7( 2): e06117. DOI: 10.1016/j.heliyon.2021.e06117. [14] LOO DT. TUNEL assay: An overview of techniques[M]// In Situ Detection of DNA Damage. New Jersey: Humana Press, 2003: 21- 30. DOI: 10.1385/1-59259-179-5: 21. [15] LI QH, ZHAO QW. Effect of continuous blood purification on inflammatory factors and vascular endothelial permeability in sepsis patients with acute respiratory distress syndrome[J]. Trauma Crit Care Med, 2021, 9( 4): 304- 306. DOI: 10.16048/j.issn.2095-5561.2021.04.16.李秋红, 赵千文. 连续性血液净化对脓毒症并发急性呼吸窘迫综合征患者炎症因子及血管内皮通透性影响[J]. 创伤与急危重病医学, 2021, 9( 4): 304- 306. DOI: 10.16048/j.issn.2095-5561.2021.04.16. [16] ZUO HZ, TIAN LJ, YANG XF, et al. Study on Logistic regression analysis of risk factors for secondary acute kidney injury in patients with sepsis[J]. J Changchun Univ Chin Med, 2023, 39( 8): 915- 920. DOI: 10.13463/j.cnki.cczyy.2023.08.021.左海忠, 田六九, 杨晓帆, 等. 脓毒症患者继发急性肾损伤危险因素Logistic 回归方程研究[J]. 长春中医药大学学报, 2023, 39( 8): 915- 920. DOI: 10.13463/j.cnki.cczyy.2023.08.021. [17] LELUBRE C, VINCENT JL. Mechanisms and treatment of organ failure in sepsis[J]. Nat Rev Nephrol, 2018, 14( 7): 417- 427. DOI: 10.1038/s41581-018-0005-7. [18] WU Y, REN JA. Role of oxidative stress in liver injury caused by sepsis[J]. Chin J Pact Surg, 2014, 34( 2): 187- 189.吴吟, 任建安. 氧化应激在脓毒症导致的肝损伤中的作用[J]. 中国实用外科杂志, 2014, 34( 2): 187- 189. [19] CERDÁ-BERNAD D, VALERO-CASES E, PASTOR JJ, et al. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action[J]. Crit Rev Food Sci Nutr, 2022, 62( 12): 3232- 3249. DOI: 10.1080/10408398.2020.1864279. [20] ABDALLA Y, ABDALLA A, HAMZA AA, et al. Safranal prevents liver cancer through inhibiting oxidative stress and alleviating inflammation[J]. Front Pharmacol, 2022, 12: 777500. DOI: 10.3389/fphar.2021.777500. [21] GUPTA M, WANI A, AHSAN AU, et al. Safranal inhibits NLRP3 inflammasome activation by preventing ASC oligomerization[J]. Toxicol Appl Pharmacol, 2021, 423: 115582. DOI: 10.1016/j.taap.2021.115582. [22] ABDALLA A, MURALI C, AMIN A. Safranal inhibits angiogenesis via targeting HIF-1α/VEGF machinery: in vitro and Ex vivo insights[J]. Front Oncol, 2022, 11: 789172. DOI: 10.3389/fonc.2021.789172. [23] XIAO Q, SUN YY, LU ZJ, et al. Protective effects of safranal on diabetic retinopathy in human microvascular endothelial cells and related pathways analyzed with transcriptome sequencing[J]. Front Endocrinol, 2022, 13: 945446. DOI: 10.3389/fendo.2022.945446. [24] ÖN ALAYUNT, AKSOY L, KARAFAKIOĞLU YS, et al. Assessment of anti-inflammatory and antioxidant properties of safranal on CCl4-induced oxidative stress and inflammation in rats[J]. An Acad Bras Cienc, 2019, 91( 2): e20181235. DOI: 10.1590/0001-3765201920181235. [25] SABIR U, IRFAN HM, ALAMGEER, et al. Reduction of hepatic steatosis, oxidative stress, inflammation, ballooning and insulin resistance after therapy with safranal in NAFLD animal model: A new approach[J]. J Inflamm Res, 2022, 15: 1293- 1316. DOI: 10.2147/JIR.S354878. [26] CAO LJ, GONG H, YAN M, et al. Research progress on Nrf2-ARE signaling pathway involved in liver disease pathological mechanism[J]. Chin Pharmacol Bull, 2015, 31( 8): 1057- 1061. DOI: 10.3969/j.issn.1001-1978.2015.08.006.曹玲娟, 龚慧, 颜苗, 等. Nrf2-ARE信号通路参与肝脏疾病病理机制研究进展[J]. 中国药理学通报, 2015, 31( 8): 1057- 1061. DOI: 10.3969/j.issn.1001-1978.2015.08.006. [27] LU XL, JIANG YY, CAO Q. The role of oxidative stress and nuclear factor erythroid 2-related factor 2 in nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2020, 36( 4): 924- 927. DOI: 10.3969/j.issn.1001-5256.2020.04.048.陆孝良, 蒋元烨, 曹勤. 氧化应激与核因子E2相关因子2在非酒精性脂肪性肝病中的作用[J]. 临床肝胆病杂志, 2020, 36( 4): 924- 927. DOI: 10.3969/j.issn.1001-5256.2020.04.048. [28] WANG TT, CHEN CY, YANG L, et al. Role of Nrf2/HO-1 signal axis in the mechanisms for oxidative stress-relevant diseases[J]. J Cent South Univ Med Sci, 2019, 44( 1): 74- 80. DOI: 10.11817/j.issn.1672-7347.2019.01.012.王甜甜, 陈淳媛, 杨雷, 等. Nrf2/HO-1信号轴在氧化应激性疾病中的机制[J]. 中南大学学报(医学版), 2019, 44( 1): 74- 80. DOI: 10.11817/j.issn.1672-7347.2019.01.012. [29] LERTNIMITPHUN P, ZHANG WH, FU WW, et al. Safranal alleviated OVA-induced asthma model and inhibits mast cell activation[J]. Front Immunol, 2021, 12: 585595. DOI: 10.3389/fimmu.2021.585595. [30] WANG HF, ZHENG B, CHE KM, et al. Protective effects of safranal on hypoxia/reoxygenation-induced injury in H9c2 cardiac myoblasts via the PI3K/AKT/GSK3β signaling pathway[J]. Exp Ther Med, 2021, 22( 6): 1400. DOI: 10.3892/etm.2021.10836. [31] XUE YR, JIN WY, XUE YC, et al. Safranal, an active constituent of saffron, ameliorates myocardial ischemia via reduction of oxidative stress and regulation of Ca2+ homeostasis[J]. J Pharmacol Sci, 2020, 143( 3): 156- 164. DOI: 10.1016/j.jphs.2020.03.005. [32] ZHANG YB, ZHAO Y, GUO JY, et al. Anticancer activity of safranal against colon carcinoma is due to induction of apoptosis and G2/M cell cycle arrest mediated by suppression of mTOR/PI3K/Akt pathway[J]. J BUON, 2021, 26( 1): 297. -



 PDF下载 ( 1990 KB)
PDF下载 ( 1990 KB)

 下载:
下载: