溶血磷脂酸(LPA)对肝癌细胞的影响及相关机制的初步探讨
DOI: 10.3969/j.issn.1001-5256.2023.11.016
-
摘要:
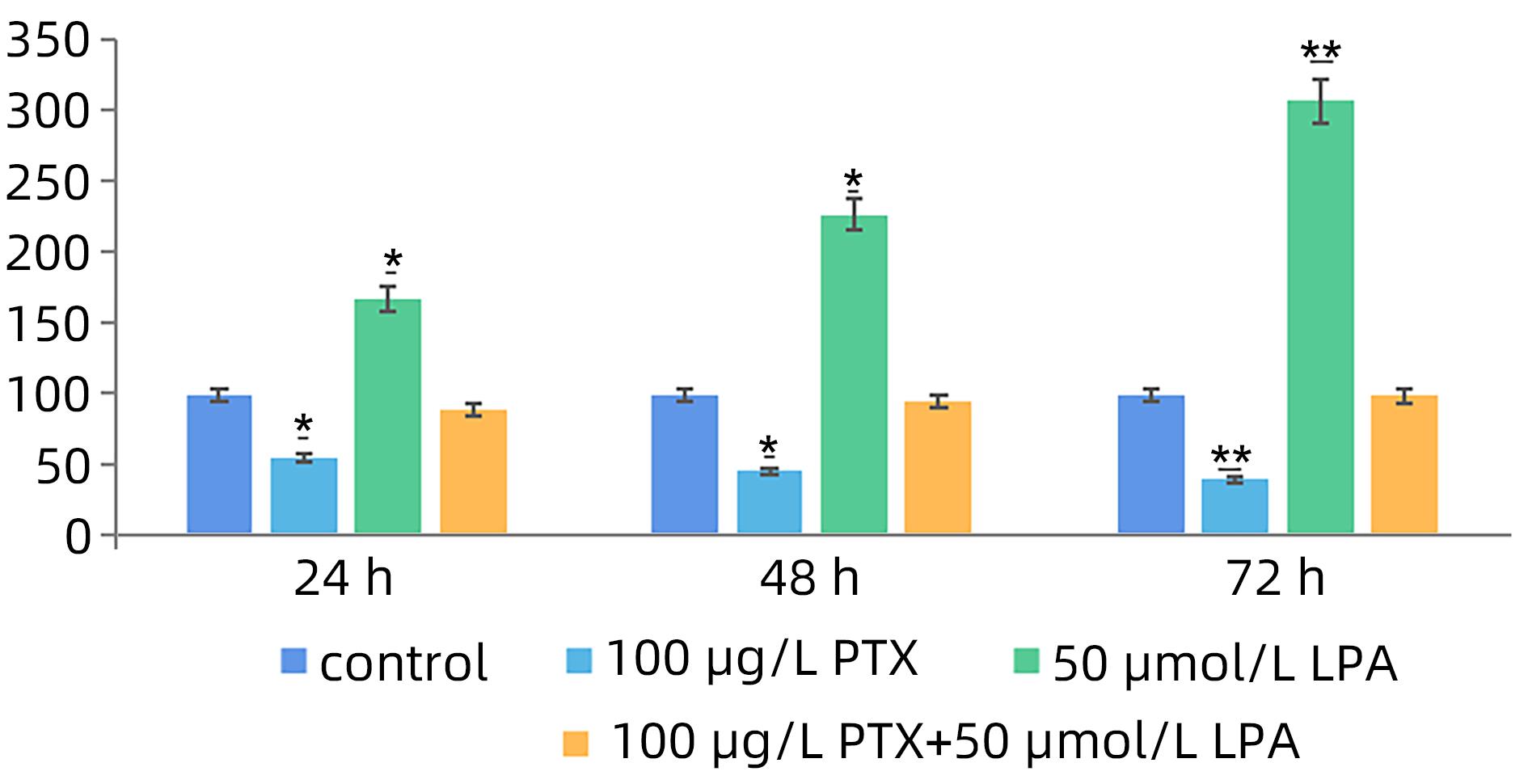
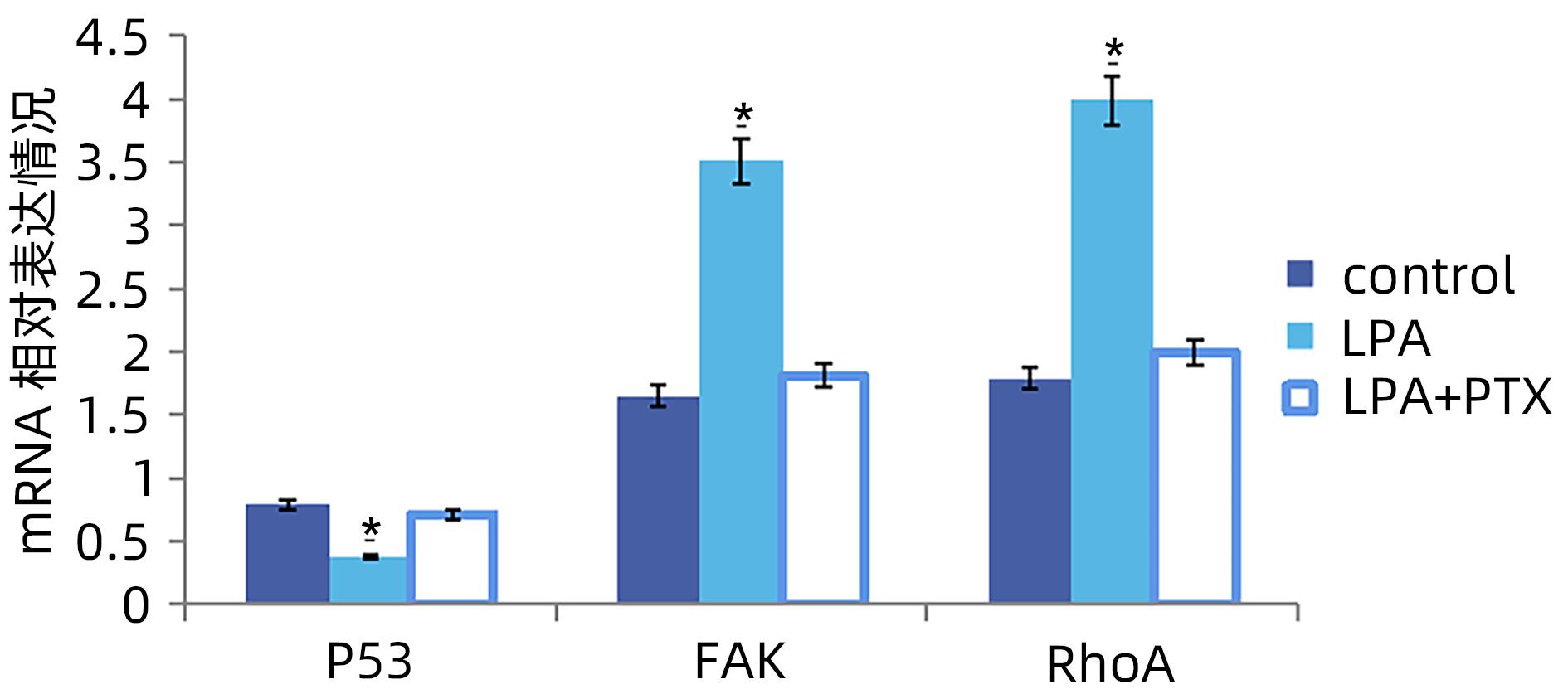
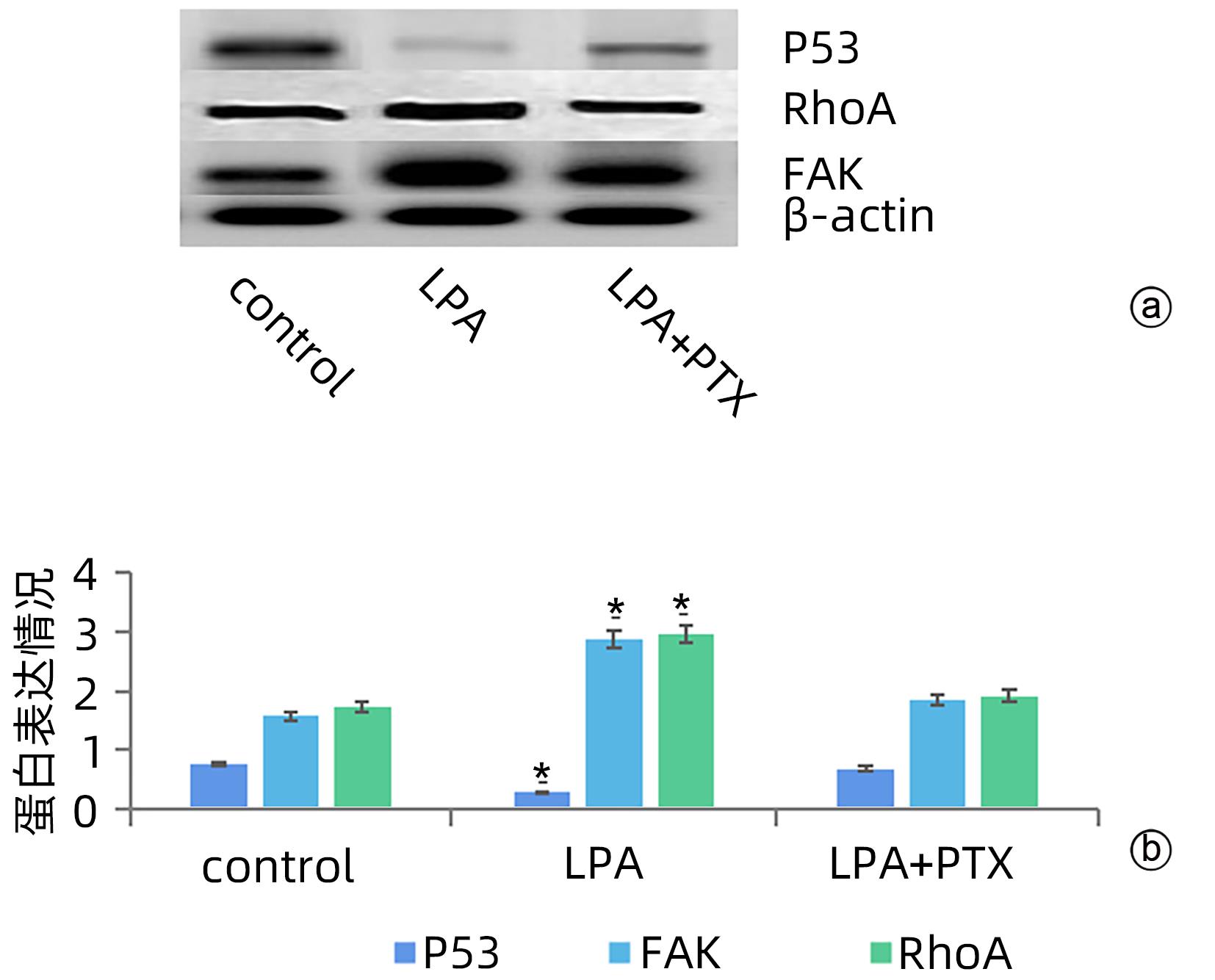
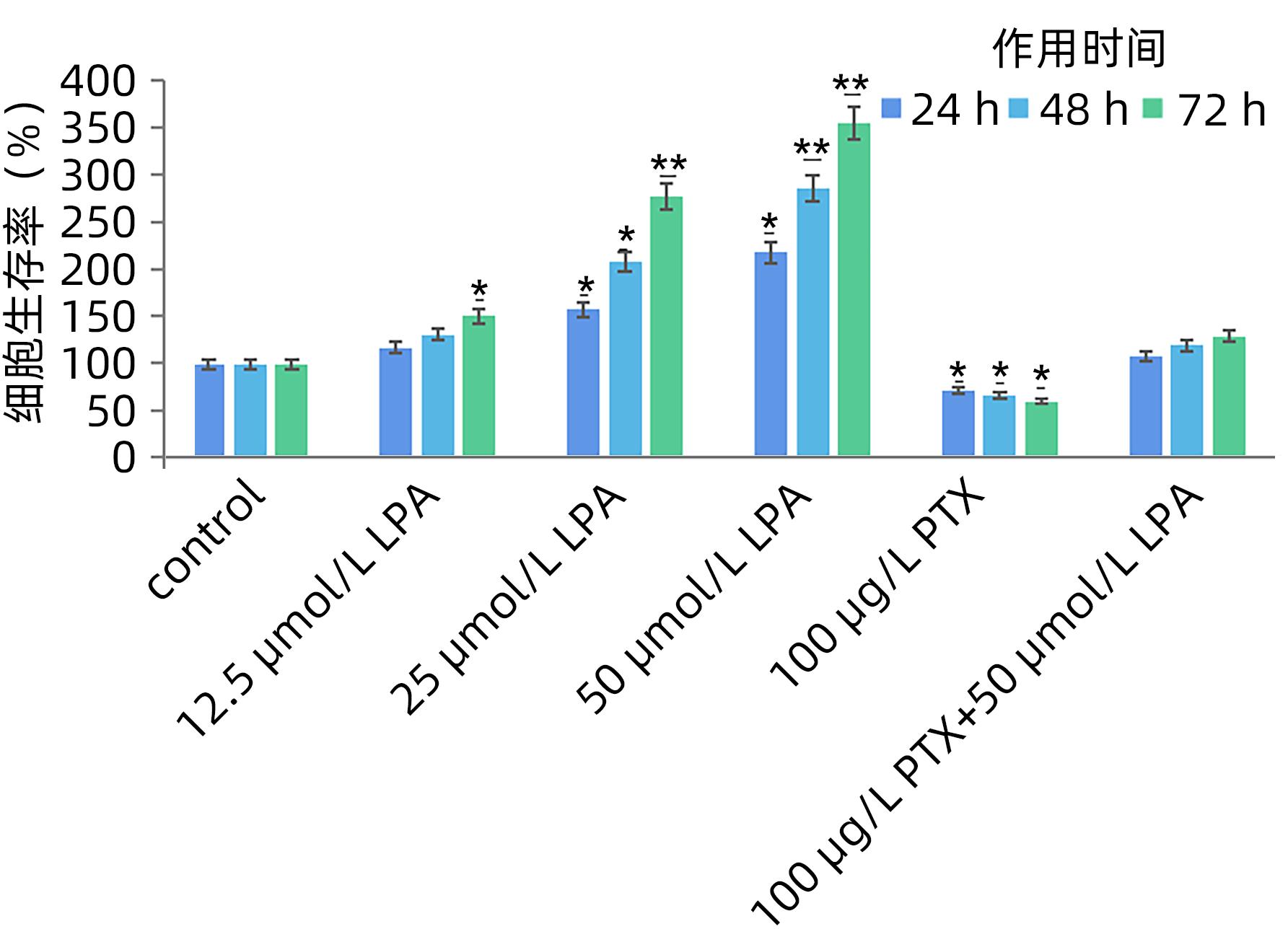
目的 探讨溶血磷脂酸(LPA)在肝癌患者中的表达情况和对肝癌恶性生物学行为的影响和调控机制。 方法 收集2016年1月—2022年12月吉林省一汽总医院收治的26例肝癌患者,28例肝硬化患者以及28例体检人群作为对照。用ELISIA方法检测伴有腹腔积液的肝癌和肝硬化患者血浆及腹腔积液中LPA含量,同时检测正常人群血浆中LPA含量,明确LPA在肝癌和肝硬化患者等不同人群中的表达差异。MTT细胞增殖实验和细胞迁移实验检测LPA和其抑制剂-百日咳毒素(PTX)对SMMC7721细胞增殖、迁移和侵袭能力的影响。为探讨LPA和其受体结合后对RhoA及其上下游的FAK、P53表达的影响,本研究采用qPCR和Western Blot法检测LPA对SMMC7721细胞P53、FAK、RhoA的mRNA和蛋白表达水平的影响。计量资料多组间样本均数比较采用单因素方差分析,两两比较采用SNK-q检验。 结果 肝癌患者血浆LPA浓度(4.99±0.55)μmol/L和腹腔积液中LPA浓度(5.19±0.63)μmol/L明显高于肝硬化患者(2.63±0.43)μmol/L、(2.91±0.46)μmol/L,肝癌患者血浆LPA亦明显高于正常人群(1.61±0.39)μmol/L,差异均有统计学意义(P值均<0.05)。细胞增殖试验显示LPA可明显促进SMMC7721细胞增殖,细胞增殖率随剂量和时间增加而增加,尤其中高剂量组增殖率明显高于对照组,差异具有统计学意义(P<0.05)。而PTX抑制其增殖能力,且具有时间依赖性,各组差异具有统计学意义(P<0.05)。72 h高剂量LPA的增殖率是对照组的3.6倍,PTX组是对照组的0.6倍,二者联合组是对照组的1.2倍。另外,LPA可增加肝癌细胞的迁袭能力,而PTX可抑制其迁移,随时间增加上述能力增加,各组差异具有统计学意义(P<0.05)。72 h高剂量LPA的迁袭率是对照组的3.09倍,PTX组是对照组的0.4倍,二者联合组是对照组的0.99倍。qPCR和Western Blot结果显示LPA处理后的SMMC7721细胞P53的mRNA和蛋白表达水平明显减少,而FAK、RhoA的mRNA和蛋白表达水平明显增加,LPA和对照组间比差异具有统计学意义(P<0.05)。 结论 在肝癌患者中LPA表达异常增高,LPA可促进肝细胞癌的增殖并增加细胞迁袭能力。同时LPA改变了P53、FAK和RhoA的表达水平,这可能与LPA促进肿瘤发生发展有关。 Abstract:Objective To investigate the expression of lysophosphatidic acid (LPA) in patients with liver cancer, as well as its influence on malignant biological behavior of liver cancer and related regulatory mechanism. Methods From January 2016 to December 2022, 26 patients with liver cancer, 28 patients with liver cirrhosis, and 28 individuals undergoing physical examination were enrolled. ELISIA was used to measure the content of LPA in plasma and peritoneal effusion of the patients with liver cancer or liver cirrhosis accompanied by peritoneal effusion, and the content of LPA was measured in plasma of the normal population at the same time, so as to clarify the difference in the expression of LPA in different populations, such as the patients with liver cancer and those with liver cirrhosis. MTT cell proliferation assay and cell migration assay were used to observe the influence of LPA and its inhibitor pertussis toxin (PTX) on the proliferation, migration, and invasion of SMMC7721 cells. In order to investigate the effect of LPA on the expression of RhoA and its upstream and downstream molecules FAK and P53 after binding to its receptor, qPCR and Western blot were used to observe the effect of LPA on the mRNA and protein expression levels of P53, FAK, and RhoA in SMMC7721 cells. A one-way analysis of variance was used for comparison of the means of continuous data between multiple groups, and the SNK-q test was used for comparison between two groups. Results Compared with the patients with liver cirrhosis, the patients with liver cancer had a significantly higher concentration of LPA in plasma (4.99±0.55 μmol/L vs 2.63±0.43 μmol/L, P<0.05) and peritoneal effusion (5.19±0.63 μmol/L vs 2.91±0.46 μmol/L, P<0.05), and the patients with liver cancer also had a significantly higher level of plasma LPA than the normal population (4.99±0.55 μmol/L vs 1.61±0.39 μmol/L, P<0.05). The cell proliferation assay showed that LPA significantly promoted the proliferation of SMMC7721 cells, and cell proliferation rate increased with the increase in dose and time; in particular, the middle-and high-dose groups had a significantly higher proliferation rate than the control group (P<0.05). PTX inhibited the proliferative capacity of SMMC7721 cells in a time-dependent manner, and there was a significant difference between the groups (P<0.05). The proliferation rate of the 72-hour high-dose LPA group was 3.6 times that of the control group, while the proliferation rate of the PTX group was 0.6 times that of the control group; the proliferation rate of the 72-hour high-dose LPA+PTX group was 1.2 times that of the control group. In addition, LPA increased the migration ability of hepatoma cells, while PTX inhibited their migration, in a time-dependent manner, and there was a significant difference between the groups (P<0.05). The migration rate of the 72-hour high-dose LPA group was 3.09 times that of the control group, while the migration rate of the PTX group was 0.4 times that of the control group; the migration rate of the 72-hour high-dose LPA+PTX group was 0.99 times that of the control group. qPCR and Western blot showed that there were significant reductions in the mRNA and protein expression levels of P53 in SMMC7721 cells after LPA treatment, while there were significant increases in the mRNA and protein expression levels of FAK and RhoA; there was a significant difference between the LPA group and the control group (P<0.05). Conclusion There is an abnormal increase in the expression of LPA in patients with liver cancer, and LPA can promote the proliferation of liver cancer cells and increase their migration ability. At the same time, LPA changes the expression levels of P53, FAK, and RhoA, which may be associated with the promotion of tumor development and progression by LPA. -
Key words:
- Liver Neoplasms /
- Lysophospholipids /
- Pertussis Toxin
-
表 1 肝癌、肝硬化患者以及正常人群LPA的表达水平
Table 1. Expression level of LPA in patients with liver cancer, liver cirrhosis and normal people
组别 LPA水平(μmol/L) 对照组 1.61±0.39 肝癌组血浆 4.99±0.551) 肝硬化组血浆 2.63±0.43 肝癌组腹腔积液 5.19±0.631) 肝硬化组腹腔积液 2.91±0.46 注:1)与对照组比较,P<0.05。 -
[1] LI X, RAMADORI P, PFISTER D, et al. The immunological and metabolic landscape in primary and metastatic liver cancer[J]. Nat Rev Cancer, 2021, 21( 9): 541- 557. DOI: 10.1038/s41568-021-00383-9. [2] XU Z, LIU Y, LIANG F. Clinical efficacy of Danshen Chuanxiongqin Injection and its effect on serum levels of LPA,Hcy and MCP-1 in patients with ischemic stroke[J]. J Changchun Univ Chin Med, 2021, 37( 1): 84- 87. DOI: 10.13463/j.cnki.cczyy.2021.01.023.许卓, 刘洋, 梁赋. 丹参川芎嗪注射液治疗缺血性脑卒中患者临床疗效及对血清LPA、Hcy、MCP-1水平的影响[J]. 长春中医药大学学报, 2021, 37( 1): 84- 87. DOI: 10.13463/j.cnki.cczyy.2021.01.023. [3] AIELLO S, CASIRAGHI F. Lysophosphatidic acid: Promoter of cancer progression and of tumor microenvironment development. A promising target for anticancer therapies?[J]. Cells, 2021, 10( 6): 1390. DOI: 10.3390/cells10061390. [4] RAY R, JANGDE N, SINGH SK, et al. Lysophosphatidic acid-RAGE axis promotes lung and mammary oncogenesis via protein kinase B and regulating tumor microenvironment[J]. Cell Commun Signal, 2020, 18( 1): 170. DOI: 10.1186/s12964-020-00666-y. [5] AMARAL RF, GERALDO LHM, EINICKER-LAMAS M, et al. Microglial lysophosphatidic acid promotes glioblastoma proliferation and migration via LPA1 receptor[J]. J Neurochem, 2021, 156( 4): 499- 512. DOI: 10.1111/jnc.15097. [6] KLYMENKO Y, BOS B, CAMPBELL L, et al. Lysophosphatidic acid modulates ovarian cancer multicellular aggregate assembly and metastatic dissemination[J]. Sci Rep, 2020, 10( 1): 10877. DOI: 10.1038/s41598-020-67565-7. [7] GNOCCHI D, CAVALLUZZI MM, MANGIATORDI GF, et al. Xanthenylacetic acid derivatives effectively target lysophosphatidic acid receptor 6 to inhibit hepatocellular carcinoma cell growth[J]. ChemMedChem, 2021, 16( 13): 2121- 2129. DOI: 10.1002/cmdc.202100032. [8] DEHGHAN M, SHAHBAZI S, SALEHNIA M. Effect of lysophosphatidic acid on the vascular endothelial growth factor expression in autotransplanted mouse ovaries encapsulated in sodium alginate[J]. J Family Reprod Health, 2021, 15( 2): 91- 98. DOI: 10.18502/jfrh.v15i2.6449. [9] XIE Y, WANG XC, WU XW, et al. Lysophosphatidic acid receptor 4 regulates osteogenic and adipogenic differentiation of progenitor cells via inactivation of RhoA/ROCK1/β-catenin signaling[J]. Stem Cells, 2020, 38( 3): 451- 463. DOI: 10.1002/stem.3128. [10] MINAMI K, UEDA N, ISHIMOTO K, et al. Cooperation of G12/13 and Gi proteins via lysophosphatidic acid receptor-2(LPA2) signaling enhances cancer cell survival to cisplatin[J]. Biochem Biophys Res Commun, 2020, 532( 3): 427- 432. DOI: 10.1016/j.bbrc.2020.08.087. [11] LEE SC, LIN KH, BALOGH A, et al. Dysregulation of lysophospholipid signaling by p53 in malignant cells and the tumor microenvironment[J]. Cell Signal, 2021, 78: 109850. DOI: 10.1016/j.cellsig.2020.109850. [12] ZHANG YZ, SHI HB, CHEN Y. Biological functions of apoptosis stimulating protein of p53 2 and its role in liver diseases[J]. J Clin Hepatol, 2018, 34( 11): 2443- 2447. DOI: 10.3969/j.issn.1001-5256.2018.11.039.张译之, 时红波, 陈煜. p53凋亡刺激蛋白2的生物学功能及其在肝病中的作用[J]. 临床肝胆病杂志, 2018, 34( 11): 2443- 2447. DOI: 10.3969/j.issn.1001-5256.2018.11.039. [13] HUANG CC, TSENG TT, LIU SC, et al. S1P increases VEGF production in osteoblasts and facilitates endothelial progenitor cell angiogenesis by inhibiting miR-16-5p expression via the c-src/FAK signaling pathway in rheumatoid arthritis[J]. Cells, 2021, 10( 8): 2168. DOI: 10.3390/cells10082168. [14] WEI YH, WANG YF, LIU NB, et al. A FAK inhibitor boosts anti-PD1 immunotherapy in a hepatocellular carcinoma mouse model[J]. Front Pharmacol, 2022, 12: 820446. DOI: 10.3389/fphar.2021.820446. [15] LIAO Y, LIU L, YANG JY, et al. ATX/LPA axis regulates FAK activation, cell proliferation, apoptosis, and motility in human pancreatic cancer cells[J]. Vitro Cell Dev Biol Anim, 2022, 58( 4): 307- 315. DOI: 10.1007/s11626-022-00660-3. [16] SUMITOMO A, SIRIWACH R, THUMKEO D, et al. LPA induces keratinocyte differentiation and promotes skin barrier function through the LPAR1/LPAR5-RHO-ROCK-SRF axis[J]. J Invest Dermatol, 2019, 139( 5): 1010- 1022. DOI: 10.1016/j.jid.2018.10.034. [17] KIM D, KIM HJ, BAEK JO, et al. Lysophosphatidic acid mediates imiquimod-induced psoriasis-like symptoms by promoting keratinocyte proliferation through LPAR1/ROCK2/PI3K/AKT signaling pathway[J]. Int J Mol Sci, 2021, 22( 19): 10777. DOI: 10.3390/ijms221910777. [18] NAKAJIMA K, OKA S, TANIKAWA T, et al. Lysophosphatidylinositol induced morphological changes and stress fiber formation through the GPR55-RhoA-ROCK pathway[J]. Int J Mol Sci, 2022, 23( 18): 10932. DOI: 10.3390/ijms231810932. [19] INABA A, HARADA H, IKEZAKI S, et al. LPA6-RhoA signals regulate junctional complexes for polarity and morphology establishment of maturation stage ameloblasts[J]. J Oral Biosci, 2022, 64( 1): 85- 92. DOI: 10.1016/j.job.2022.01.004. [20] BUTERA A, ROY M, ZAMPIERI C, et al. p53-driven lipidome influences non-cell-autonomous lysophospholipids in pancreatic cancer[J]. Biol Direct, 2022, 17( 1): 6. DOI: 10.1186/s13062-022-00319-9. -



 PDF下载 ( 837 KB)
PDF下载 ( 837 KB)

 下载:
下载: