戊型肝炎高风险人群的防治进展
DOI: 10.3969/j.issn.1001-5256.2023.11.002
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:朱月萍负责查阅文献,起草文章;朱传武负责拟定课题,设计写作思路,指导撰写文章并最后定稿。
Advances in the prevention and treatment of the high-risk population for hepatitis E
-
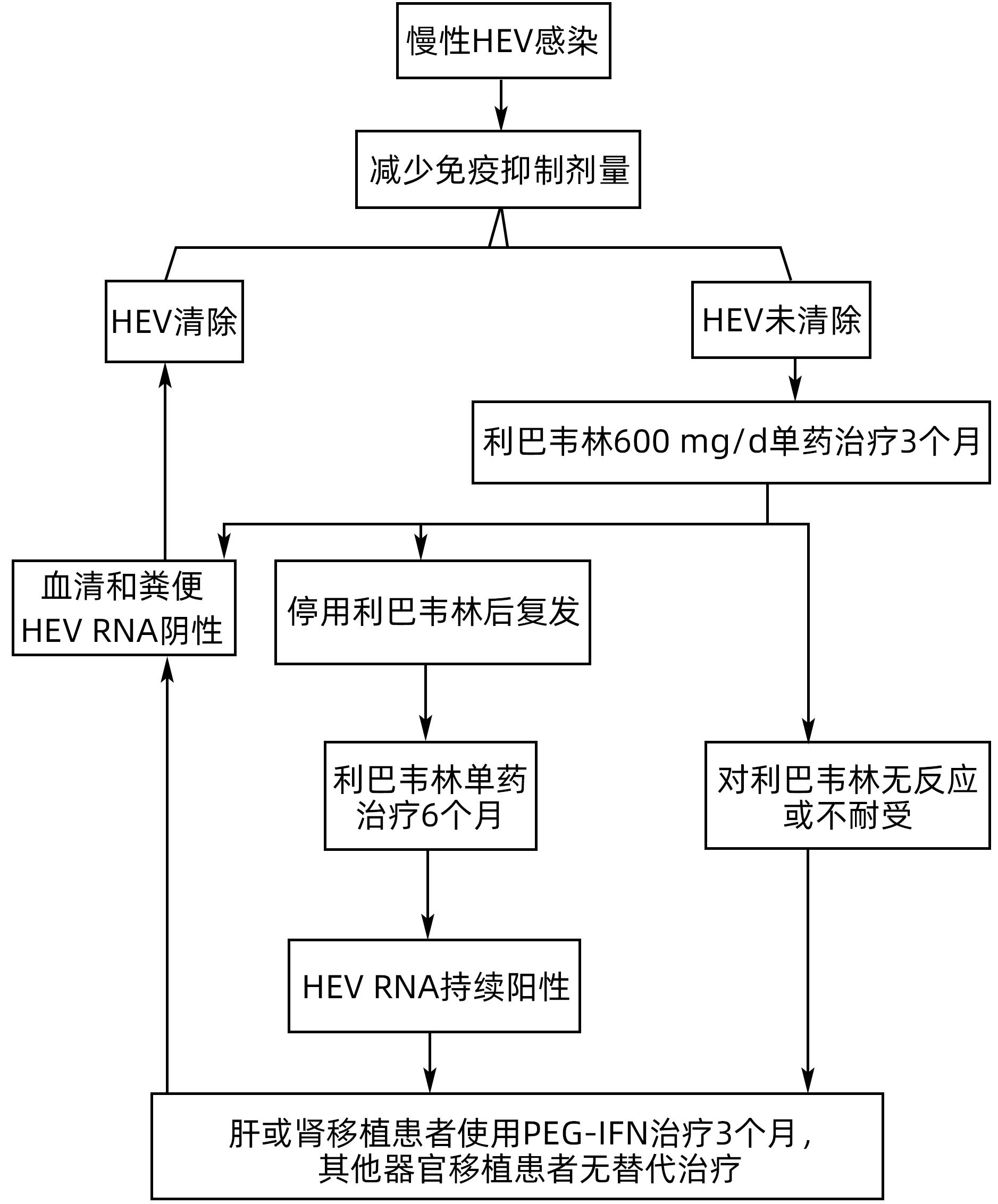
摘要: 戊型肝炎病毒(HEV)感染是引起急性病毒性肝炎的最常见病因之一。大多数HEV感染是无症状的,机体能够自发清除病毒。孕妇、老年人、免疫功能低下人群、慢性肝病患者以及与HEV感染动物密切接触的人员是HEV感染的高风险人群。重组戊型肝炎疫苗HEV 239是目前唯一获批的戊型肝炎疫苗,具有短期和长期的保护效力,该疫苗安全性高,不良反应少,高风险人群应优先接种。免疫功能低下者感染HEV可发生慢性感染。利巴韦林和干扰素是目前治疗HEV感染最常用的抗病毒药物。然而,对于利巴韦林或干扰素治疗有禁忌证或治疗无应答的患者,尚需开发安全有效的新型抗病毒药物。Abstract: Hepatitis E virus (HEV) infection is one of the most common causes of acute viral hepatitis. Most patients with HEV infection are asymptomatic and the virus can be spontaneously eliminated. Pregnant women, the elderly, immunocompromised populations, patients with chronic liver disease, and individuals in close contact with HEV-infected animals are at a high risk for HEV infection. The recombinant hepatitis E vaccine HEV 239 is the only approved hepatitis E vaccine, with both short- and long-term protective efficacy. This vaccine has a favorable safety profile with few adverse events, and the high-risk populations should be given the priority to receive such vaccination. Immunocompromised individuals may develop chronic HEV infection. Ribavirin and interferon are currently the most commonly used antiviral drugs for the treatment of HEV infection; however, it still needs to develop safe and effective novel antiviral drugs for patients with contraindications to ribavirin or interferon or those who have no response to such therapy.
-
Key words:
- Hepatitis E /
- High-Risk Population /
- Viral Hepatitis Vaccines /
- Therapeutics
-
[1] World Health Organization(WHO). Hepatitis E[EB/OL].( 2019-7-8). https://www.who.int/news-room/fact-sheets/detail/hepatitis-e. https://www.who.int/news-room/fact-sheets/detail/hepatitis-e [2] National Health Commission of the People’s Republic of China. The epidemic situation of statutory reporting infectious diseases in 2021[EB/OL]. http://www.nhc.gov.cn/jkj/s3578/202204/4fd88a291d914abf8f7a91f6333567e1.shtml. http://www.nhc.gov.cn/jkj/s3578/202204/4fd88a291d914abf8f7a91f6333567e1.shtml国家卫生健康委员会. 2021年全国法定传染病疫情概况[EB/OL]. http://www.nhc.gov.cn/jkj/s3578/202204/4fd88a291d914abf8f7a91f6333567e1.shtml. http://www.nhc.gov.cn/jkj/s3578/202204/4fd88a291d914abf8f7a91f6333567e1.shtml [3] HOOFNAGLE JH, NELSON KE, PURCELL RH. Hepatitis E[J]. N Engl J Med, 2012, 367( 13): 1237- 1244. DOI: 10.1056/nejmra1204512. [4] MA ZR, DE MAN RA, KAMAR N, et al. Chronic hepatitis E: Advancing research and patient care[J]. J Hepatol, 2022, 77( 4): 1109- 1123. DOI: 10.1016/j.jhep.2022.05.006. [5] SMITH DB, IZOPET J, NICOT F, et al. Update: Proposed reference sequences for subtypes of hepatitis E virus(species Orthohepevirus A)[J]. J Gen Virol, 2020, 101( 7): 692- 698. DOI: 10.1099/jgv.0.001435. [6] ASLAN AT, BALABAN HY. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment[J]. World J Gastroenterol, 2020, 26( 37): 5543- 5560. DOI: 10.3748/wjg.v26.i37.5543. [7] PISCHKE S, WEDEMEYER H. Hepatitis E virus infection: Multiple faces of an underestimated problem[J]. J Hepatol, 2013, 58( 5): 1045- 1046. DOI: 10.1016/j.jhep.2012.12.013. [8] KAMAR N, SELVES J, MANSUY JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients[J]. N Engl J Med, 2008, 358( 8): 811- 817. DOI: 10.1056/nejmoa0706992. [9] DALTON HR, BENDALL RP, KEANE FE, et al. Persistent carriage of hepatitis E virus in patients with HIV infection[J]. N Engl J Med, 2009, 361( 10): 1025- 1027. DOI: 10.1056/NEJMc0903778. [10] VON FELDEN J, ALRIC L, PISCHKE S, et al. The burden of hepatitis E among patients with haematological malignancies: A retrospective European cohort study[J]. J Hepatol, 2019, 71( 3): 465- 472. DOI: 10.1016/j.jhep.2019.04.022. [11] KAMAR N, GARROUSTE C, HAAGSMA EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants[J]. Gastroenterology, 2011, 140( 5): 1481- 1489. DOI: 10.1053/j.gastro.2011.02.050. [12] GETSUWAN S, PASOMSUB E, YUTTHANAKARNWIKOM P, et al. Seroprevalence of hepatitis E virus after pediatric liver transplantation[J]. J Trop Pediatr, 2023, 69( 2): fmad011. DOI: 10.1093/tropej/fmad011. [13] LHOMME S, LEGRAND-ABRAVANEL F, KAMAR N, et al. Screening, diagnosis and risks associated with Hepatitis E virus infection[J]. Expert Rev Anti Infect Ther, 2019, 17( 6): 403- 418. DOI: 10.1080/14787210.2019.1613889. [14] LAMPEJO T, CURTIS C, IJAZ S, et al. Nosocomial transmission of hepatitis E virus and development of chronic infection: The wider impact of COVID-19[J]. J Clin Virol, 2022, 148: 105083. DOI: 10.1016/j.jcv.2022.105083. [15] GENG YS, ZHANG HX, HUANG WJ, et al. Persistent hepatitis e virus genotype 4 infection in a child with acute lymphoblastic leukemia[J]. Hepat Mon, 2014, 14( 1): e15618. DOI: 10.5812/hepatmon.15618. [16] LENGLART A, CHAPPÉ C, GRULOIS I, et al. Hepatitis E virus infection in pediatric oncology[J]. J Pediatr Hematol Oncol, 2023, 45( 1): e150- e153. DOI: 10.1097/MPH.0000000000002539. [17] European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection[J]. J Hepatol, 2012, 57( 1): 167- 185. [18] NASIR M, WU GY. HEV and HBV dual infection: A review[J]. J Clin Transl Hepatol, 2020, 8( 3): 313- 321. DOI: 10.14218/JCTH.2020.00030. [19] XIONG LS, CUI SF, ZHOU JG, et al. Detection and analysis of HAV-HEV, HGV infection in patients with viral hepatitis[J]. Chin J Hepatol, 2004, 12( 7): 395- 396. DOI: 10.3760/j.issn: 1007-3418.2004.07.004.熊良仕, 崔素芬, 周京国, 等. 各型肝炎病毒单纯及重叠感染的研究[J]. 中华肝脏病杂志, 2004, 12( 7): 395- 396. DOI: 10.3760/j.issn:1007-3418.2004.07.004. [20] TSENG TC, LIU CJ, CHANG CT, et al. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis[J]. J Hepatol, 2020, 72( 6): 1105- 1111. DOI: 10.1016/j.jhep.2020.01.012. [21] ZHAO H, YE WY, YU X, et al. Hepatitis E virus superinfection impairs long-term outcome in hospitalized patients with hepatitis B virus-related decompensated liver cirrhosis[J]. Ann Hepatol, 2023, 28( 2): 100878. DOI: 10.1016/j.aohep.2022.100878. [22] CHOW CW, TSANG SWC, TSANG OTY, et al. Comparison of acute hepatitis E infection outcome in patients with and without chronic hepatitis B infection: A 10 year retrospective study in three regional hospitals in Hong Kong[J]. J Clin Virol, 2014, 60( 1): 4- 10. DOI: 10.1016/j.jcv.2014.01.024. [23] CHEN C, ZHANG SY, ZHANG DD, et al. Clinical features of acute hepatitis E super-infections on chronic hepatitis B[J]. World J Gastroenterol, 2016, 22( 47): 10388- 10397. DOI: 10.3748/wjg.v22.i47.10388. [24] JILANI N, DAS BC, HUSAIN SA, et al. Hepatitis E virus infection and fulminant hepatic failure during pregnancy[J]. J Gastroenterol Hepatol, 2007, 22( 5): 676- 682. DOI: 10.1111/j.1440-1746.2007.04913.x. [25] KHUROO MS, Aetiology KAMILI S., clinical course and outcome of sporadic acute viral hepatitis in pregnancy[J]. J Viral Hepat, 2003, 10( 1): 61- 69. DOI: 10.1046/j.1365-2893.2003.00398.x. [26] DI BARTOLOMEO S, CARUBBI F, CIPRIANI P. Hepatitis E virus and rheumatic diseases: What do rheumatologists need to know?[J]. BMC Rheumatol, 2020, 4: 51. DOI: 10.1186/s41927-020-00149-0. [27] KAR P, SENGUPTA A. A guide to the management of hepatitis E infection during pregnancy[J]. Expert Rev Gastroenterol Hepatol, 2019, 13( 3): 205- 211. DOI: 10.1080/17474124.2019.1568869. [28] BOSE PD, DAS BC, KUMAR A, et al. High viral load and deregulation of the progesterone receptor signaling pathway: Association with hepatitis E-related poor pregnancy outcome[J]. J Hepatol, 2011, 54( 6): 1107- 1113. DOI: 10.1016/j.jhep.2010.08.037. [29] GOUILLY J, CHEN Q, SIEWIERA J, et al. Genotype specific pathogenicity of hepatitis E virus at the human maternal-fetal interface[J]. Nat Commun, 2018, 9( 1): 4748. DOI: 10.1038/s41467-018-07200-2. [30] BHATIA V, SINGHAL A, PANDA SK, et al. A 20-year single-center experience with acute liver failure during pregnancy: Is the prognosis really worse?[J]. Hepatology, 2008, 48( 5): 1577- 1585. DOI: 10.1002/hep.22493. [31] SHARMA S, KUMAR A, KAR P, et al. Risk factors for vertical transmission of hepatitis E virus infection[J]. J Viral Hepat, 2017, 24( 11): 1067- 1075. DOI: 10.1111/jvh.12730. [32] RIVERO-JUAREZ A, FRIAS M, RODRIGUEZ-CANO D, et al. Isolation of hepatitis E virus from breast milk during acute infection[J]. Clin Infect Dis, 2016, 62( 11): 1464. DOI: 10.1093/cid/ciw186. [33] Chinese Society of Hepatology, Chinese Medical Association. Consensus on prevention and treatment of hepatitis E[J]. Chin J Hepatol, 2022, 30( 8): 820- 831. DOI: 10.3760/cma.j.cn501113-20220729-00401.中华医学会肝病学分会. 戊型肝炎防治共识[J]. 中华肝脏病杂志, 2022, 30( 8): 820- 831. DOI: 10.3760/cma.j.cn501113-20220729-00401. [34] CORDES AK, GOUDEVA L, LÜTGEHETMANN M, et al. Risk of transfusion-transmitted hepatitis E virus infection from pool-tested platelets and plasma[J]. J Hepatol, 2022, 76( 1): 46- 52. DOI: 10.1016/j.jhep.2021.08.018. [35] BOLAND F, MARTINEZ A, POMEROY L, et al. Blood donor screening for hepatitis E virus in the European union[J]. Transfus Med Hemother, 2019, 46( 2): 95- 103. DOI: 10.1159/000499121. [36] ZHU FC, ZHANG J, ZHANG XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: A large-scale, randomised, double-blind placebo-controlled, phase 3 trial[J]. Lancet, 2010, 376( 9744): 895- 902. DOI: 10.1016/S0140-6736(10)61030-6. [37] ZHANG J, ZHANG XF, HUANG SJ, et al. Long-term efficacy of a hepatitis E vaccine[J]. N Engl J Med, 2015, 372( 10): 914- 922. DOI: 10.1056/NEJMoa1406011. [38] ØVERBØ J, AZIZ A, ZAMAN K, et al. Immunogenicity and safety of a two-dose regimen with hepatitis E virus vaccine in healthy adults in rural Bangladesh: A randomized, double-blind, controlled, phase 2/pilot trial[J]. Vaccine, 2023, 41( 5): 1059- 1066. DOI: 10.1016/j.vaccine.2022.12.064. [39] WANG KH, ZHOU LZ, ZHANG X, et al. Hepatitis E vaccine candidate harboring a non-particulate immunogen of E2 fused with CRM197 fragment A[J]. Antiviral Res, 2019, 164: 154- 161. DOI: 10.1016/j.antiviral.2019.02.013. [40] GU Y, TANG XH, ZHANG X, et al. Structural basis for the neutralization of hepatitis E virus by a cross-genotype antibody[J]. Cell Res, 2015, 25( 5): 604- 620. DOI: 10.1038/cr.2015.34. [41] CHEN ZH, WEI JF, JIANG L, et al. Case Report: Chronic hepatitis E in a hematopoietic stem cell transplant recipient: The first report of hepatitis E virus genotype 4 causing chronic infection in a non-solid organ recipient[J]. Front Immunol, 2022, 13: 954697. DOI: 10.3389/fimmu.2022.954697. [42] ZHANG HY, RAO HY, WANG YJ, et al. Evaluation of an antigen assay for diagnosing acute and chronic hepatitis E genotype 4 infection[J]. J Gastroenterol Hepatol, 2019, 34( 2): 458- 465. DOI: 10.1111/jgh.14405. [43] ANKCORN M, SAID B, MORGAN D, et al. Persistent hepatitis E virus infection across England and Wales 2009-2017: Demography, virology and outcomes[J]. J Viral Hepat, 2021, 28( 2): 420- 430. DOI: 10.1111/jvh.13424. [44] KAMAR N, ROSTAING L, ABRAVANEL F, et al. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation[J]. Clin Infect Dis, 2010, 50( 5): e30- e33. DOI: 10.1086/650488. [45] GALLACHER J, TAHA Y, SILVA FILIPE A DA, et al. Ledipasvir/sofosbuvir and ribavirin for the treatment of ribavirin-refractory persistent hepatitis E virus infection[J]. IDCases, 2023, 32: e01741. DOI: 10.1016/j.idcr.2023.e01741. [46] CORNBERG M, PISCHKE S, MÜLLER T, et al. Sofosbuvir monotherapy fails to achieve HEV RNA elimination in patients with chronic hepatitis E-The HepNet SofE pilot study[J]. J Hepatol, 2020, 73( 3): 696- 699. DOI: 10.1016/j.jhep.2020.05.020. [47] TAVITIAN S, PERON JM, HUGUET F, et al. Ribavirin for chronic hepatitis prevention among patients with hematologic malignancies[J]. Emerg Infect Dis, 2015, 21( 8): 1466- 1469. DOI: 10.3201/eid2108.150199. [48] ZHANG F, XU LD, ZHANG Q, et al. Targeting proteostasis of the HEV replicase to combat infection in preclinical models[J]. J Hepatol, 2023, 78( 4): 704- 716. DOI: 10.1016/j.jhep.2022.12.010. [49] KINAST V, BURKARD TL, TODT D, et al. Hepatitis E virus drug development[J]. Viruses, 2019, 11( 6): 485. DOI: 10.3390/v11060485. -



 PDF下载 ( 703 KB)
PDF下载 ( 703 KB)

 下载:
下载:


