人工合成多肽cSN50.1对肝癌细胞HepG2恶性行为的影响及其机制
DOI: 10.3969/j.issn.1001-5256.2023.10.014
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:辛华、栾海艳负责课题设计,资料分析,撰写论文;单洪超参与收集数据,修改论文;阮洋、杨鑫妍负责拟定写作思路,指导撰写文章并最后定稿。
Effect of synthetic peptide cSN50.1 on the malignant behavior of hepatocellular carcinoma HepG2 cells and its mechanism
-
摘要:
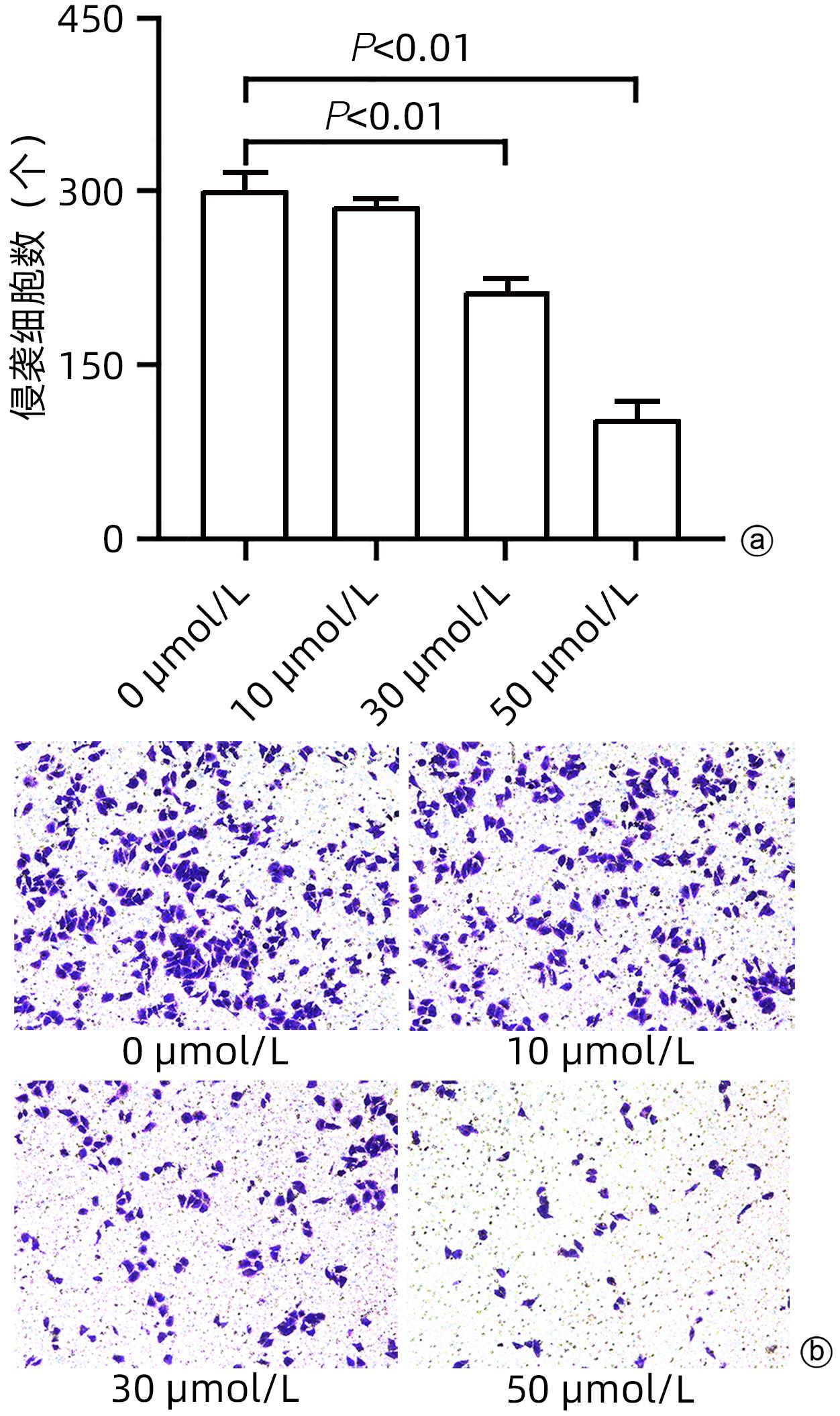
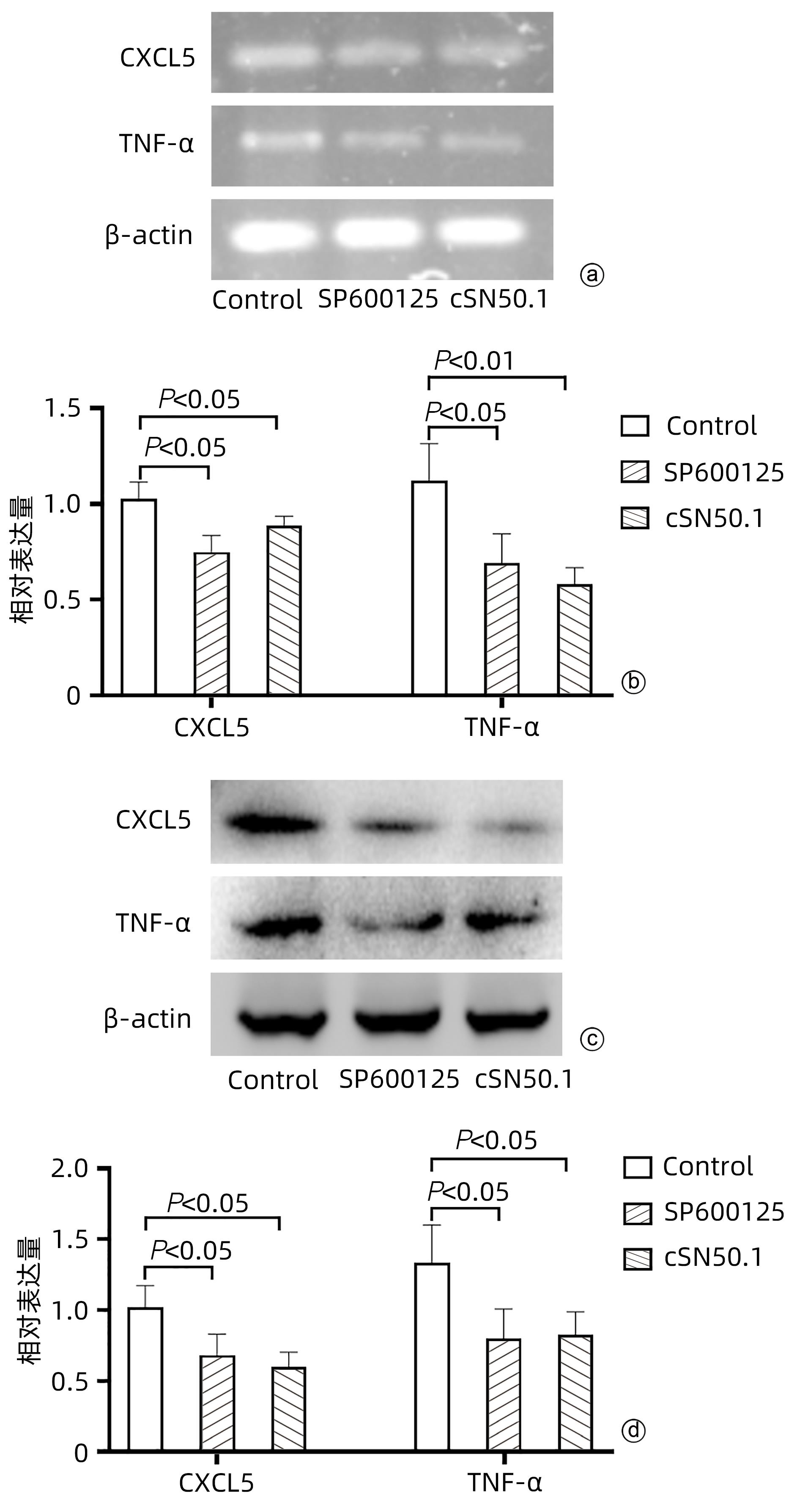
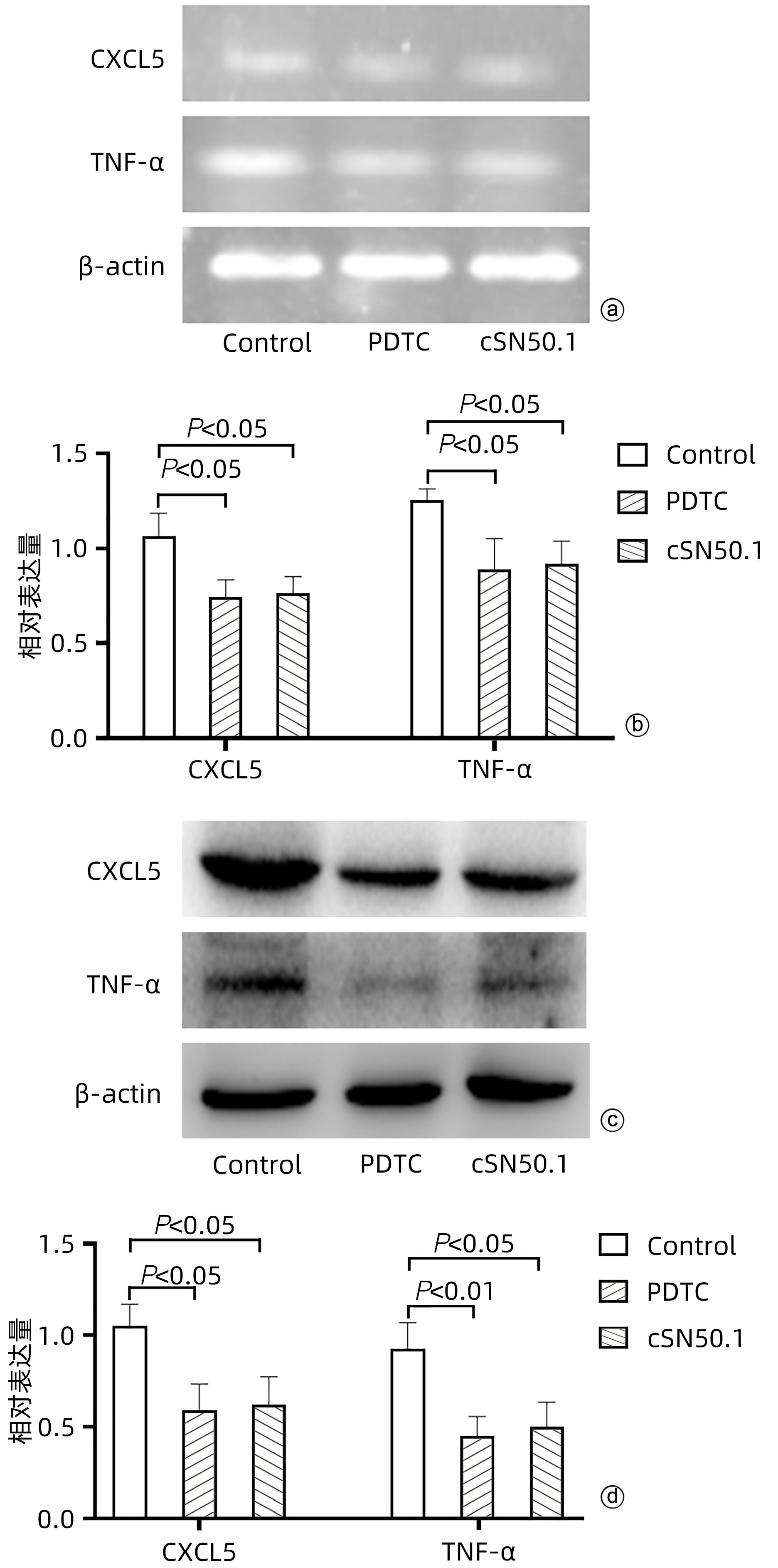
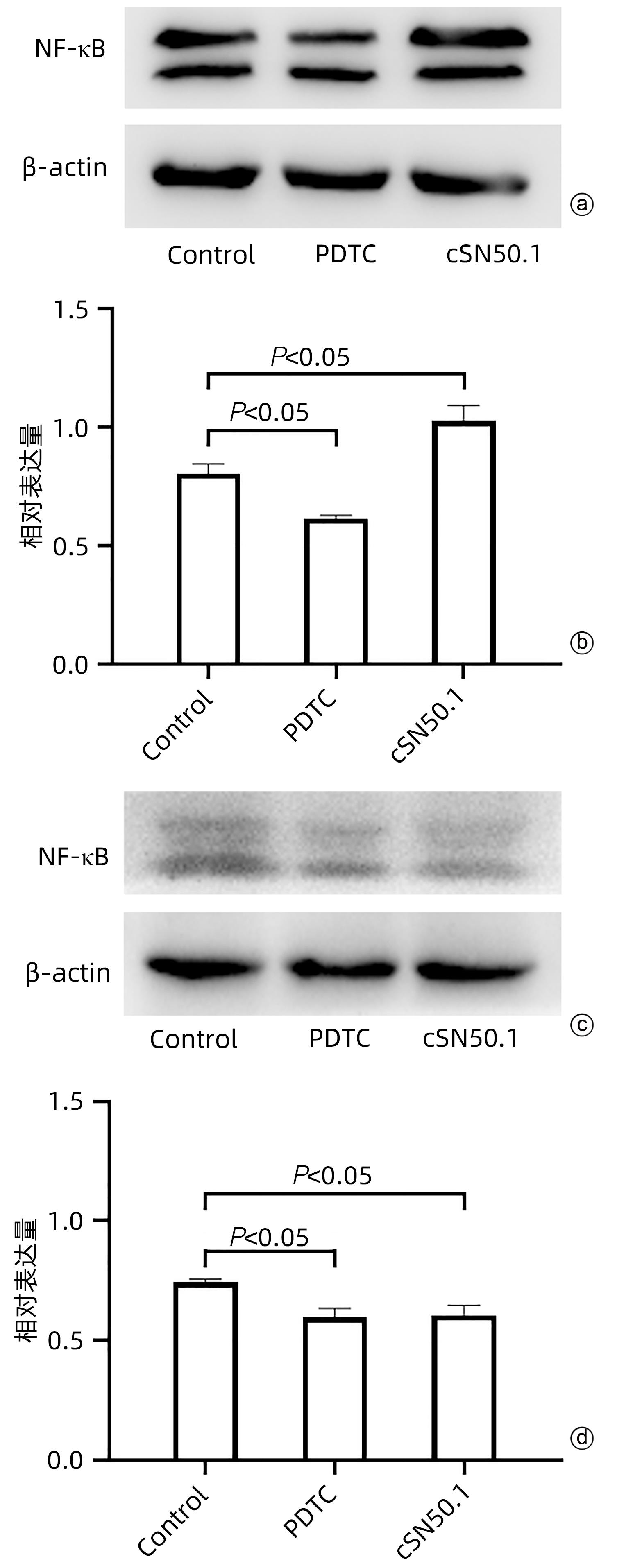
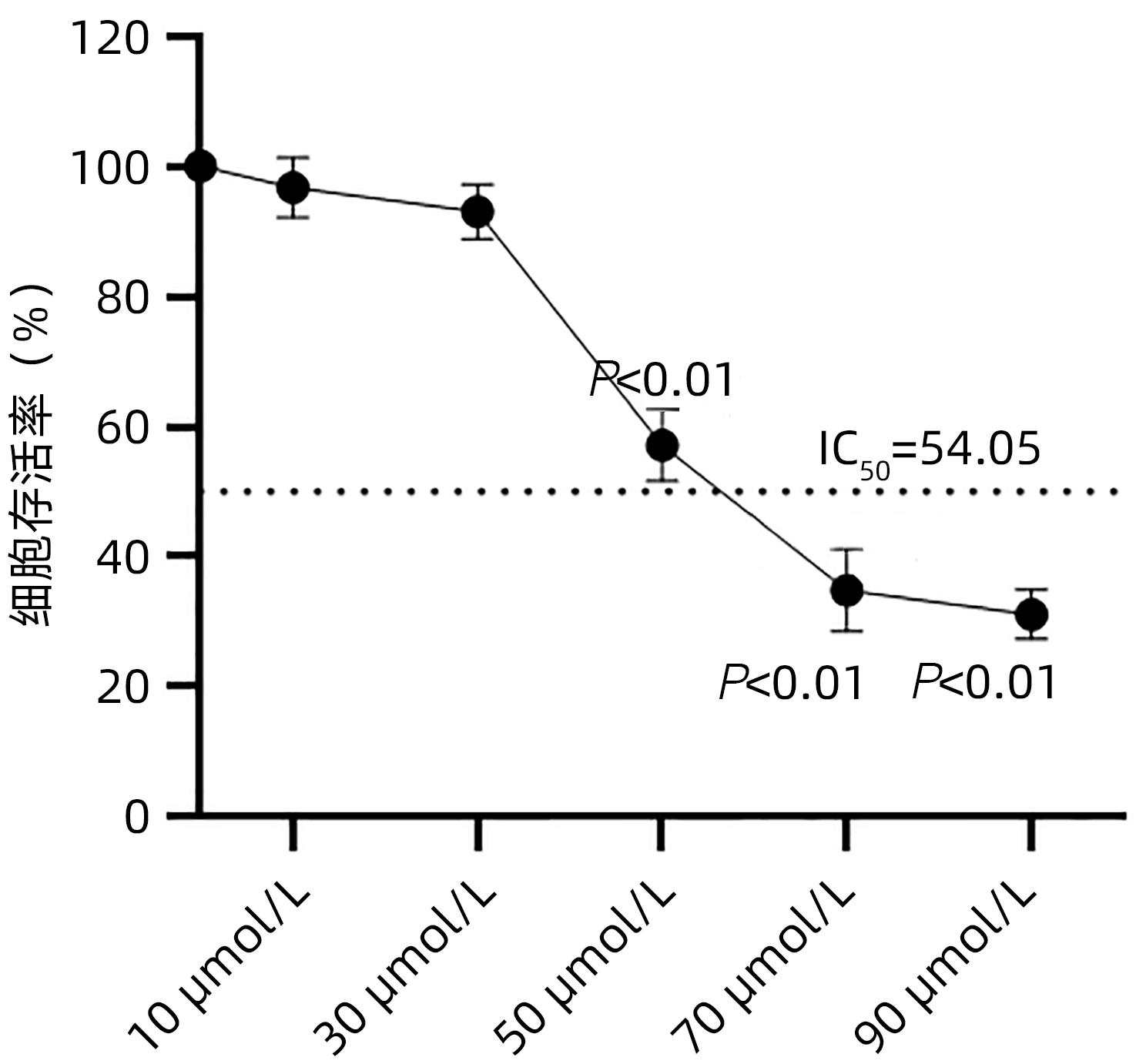
目的 探讨cSN50.1对HepG2细胞增殖、迁移、侵袭和集落形成能力的影响及机制。 方法 将HepG2细胞分为6组:cSN50.1 0 μmol/L、10 μmol/L、30 μmol/L、50 μmol/L、70 μmol/L、90 μmol/L组,采用CCK-8实验研究不同浓度cSN50.1对HepG2细胞增殖的影响,并计算半数抑制浓度(IC50);将HepG2细胞分为4组:cSN50.1 0 μmol/L、10 μmol/L、30 μmol/L、50 μmol/L,采用细胞划痕、Transwell和细胞克隆实验研究不同浓度cSN50.1对HepG2细胞迁移、侵袭和集落形成能力的影响;将HepG2细胞分为3组:Control组、SP600125组(AP-1信号通路抑制剂)和cSN50.1组,研究AP-1信号通路在cSN50.1对肝癌细胞作用中的影响,采用RT-PCR和Western Blot检测CXCL5和TNF-α的表达以及细胞质和细胞核中c-Jun蛋白的表达;将HepG2细胞分为3组:Control组、PDTC组(NF-κB信号通路抑制剂)和cSN50.1组,研究NF-κB信号通路在cSN50.1对肝癌细胞作用中的影响,采用RT-PCR和Western Blot检测CXCL5和TNF-α的表达以及细胞质和细胞核中NF-κB蛋白的表达。多组间比较采用单因素方差分析,进一步两两比较采用SNK-q检验。 结果 与0 μmol/L相比,10 μmol/L组的增殖、迁移、侵袭和集落形成能力无明显变化(P值均>0.05);30 μmol/L组的增殖能力无明显变化(P>0.05),迁移、侵袭和集落形成能力均明显降低(P值均<0.05);50 μmol/L组的增殖、迁移、侵袭和集落形成能力均明显降低(P值均<0.01);70 μmol/L和90 μmol/L组的细胞增殖能力均明显降低(P值均<0.01),但细胞存活率低于50%。与Control组相比,SP600125组、PDTC组和cSN50.1组中CXCL5和TNF-α的基因和蛋白表达均明显降低(P值均<0.05)。与Control组相比,SP600125组、PDTC组和cSN50.1组中细胞核蛋白c-Jun和NF-κB表达均明显降低(P值均<0.05),SP600125组和PDTC组中细胞质蛋白c-Jun和NF-κB表达均明显降低(P值均<0.05),cSN50.1组中细胞质蛋白c-Jun和NF-κB表达明显增高(P<0.05)。 结论 cSN50.1可以抑制肝癌细胞的恶性行为,可抑制肝癌细胞中c-Jun和NF-κB的入核转运来降低CXCL5和TNF-α的表达。 Abstract:Objective To investigate the effect of cSN50.1 on the proliferation, migration, invasion, and colony formation of HepG2 cells and its mechanism. Methods HepG2 cells were divided into cSN50.1 0 μmol/L, cSN50.1 10 μmol/L, cSN50.1 30 μmol/L, cSN50.1 50 μmol/L, cSN50.1 70 μmol/L, and cSN50.1 90 μmol/L groups, and CCK-8 assay was used to investigate the effect of different concentrations of cSN50.1 on the proliferation of HepG2 cells and calculate half-maximal inhibitory concentration (IC50). HepG2 cells were divided into cSN50.1 0 μmol/L, cSN50.1 10 μmol/L, cSN50.1 30 μmol/L, and cSN50.1 50 μmol/L groups, and wound healing assay, Transwell assay, and colony-forming assay were used to investigate the effect of different concentrations of cSN50.1 on the migration, invasion, and colony formation of HepG2 cells. HepG2 cells were divided into Control group, SP600125 group (an inhibitor of the AP-1 signaling pathway), and cSN50.1 group to investigate the influence of the AP-1 signaling pathway on the effect of cSN50.1 on hepatocellular carcinoma cells, and RT-PCR and Western Blot were used to measure the expression of CXCL5, TNF-α, and c-Jun protein in cytoplasm and nucleus. HepG2 cells were divided into Control group, PDTC group (an inhibitor of the NF-κB signaling pathway), and cSN50.1 group to investigate the influence of the NF-κB signaling pathway on the effect of cSN50.1 on hepatocellular carcinoma cells, and RT-PCR and Western Blot were used to measure the expression of CXCL5, TNF-α, and NF-κB protein in cytoplasm and nucleus. A one-way analysis of variance was used for comparison between multiple groups, and the SNK-q test was used for further comparison between two groups. Results Compared with the 0 μmol/L group, the 10 μmol/L group had no significant changes in proliferation, migration, invasion, and colony formation abilities (P >0.05); the 30 μmol/L group had no significant change in proliferation ability (P>0.05), but with significant reductions in migration, invasion, and colony formation abilities (P<0.05); the 50 μmol/L group had significant reductions in proliferation, migration, invasion, and colony formation abilities (all P<0.01); the 70 μmol/L and 90 μmol/L groups had a significant reduction in cell proliferation ability (P<0.01), but with a cell survival rate of below 50%. Compared with the Control group, the SP600125, PDTC, and cSN50.1 groups had significant reductions in the mRNA and protein expression levels of CXCL5 and TNF-α (all P<0.05). Compared with the Control group, the SP600125 group, the PDTC group, and the cSN50.1 group had a significant reduction in nuclear protein of c-Jun and NF-κB expression (P<0.05); the SP600125 group and the PDTC group had a significant reduction in cytoplasmic protein of c-Jun and NF-κB expression (P<0.05); the cSN50.1 group had a significant increase in cytoplasmic protein of c-Jun and NF-κB expression (P<0.05). Conclusion This study shows that cSN50.1 can inhibit the malignant behavior of hepatocellular carcinoma cells and reduce the expression of CXCL5 and TNF-α by inhibiting the nuclear import of c-Jun and NF-κB in hepatocellular carcinoma cells. -
-
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68( 6): 394- 424. DOI: 10.3322/caac.21492. [2] 2019 Under- GBD 5 Mortality Collaborators. Global, regional, and national progress towards Sustainable Development Goal 3.2 for neonatal and child health: All-cause and cause-specific mortality findings from the Global Burden of Disease Study 2019[J]. Lancet, 2021, 398( 10303): 870- 905. DOI: 10.1016/S0140-6736(21)01207-1. [3] VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380( 15): 1450- 1462. DOI: 10.1056/nejmra1713263. [4] LI X, RAMADORI P, PFISTER D, et al. The immunological and metabolic landscape in primary and metastatic liver cancer[J]. Nat Rev Cancer, 2021, 21( 9): 541- 557. DOI: 10.1038/s41568-021-00383-9. [5] LEE G, HAN SB, LEE JH, et al. Cancer mechanobiology: Microenvironmental sensing and metastasis[J]. ACS Biomater Sci Eng, 2019, 5( 8): 3735- 3752. DOI: 10.1021/acsbiomaterials.8b01230. [6] VEACH RA, LIU Y, ZIENKIEWICZ J, et al. Survival, bacterial clearance and thrombocytopenia are improved in polymicrobial sepsis by targeting nuclear transport shuttles[J]. PLoS One, 2017, 12( 6): e0179468. DOI: 10.1371/journal.pone.0179468. [7] ALTEKRUSE SF, MCGLYNN KA, REICHMAN ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005[J]. J Clin Oncol, 2009, 27( 9): 1485- 1491. DOI: 10.1200/JCO.2008.20.7753. [8] SCHLEIN LJ, THAMM DH. Review: NF-kB activation in canine cancer[J]. Vet Pathol, 2022, 59( 5): 724- 732. DOI: 10.1177/03009858221092017. [9] YANG Y, LI TF, HUANG C. Research progress of tumor stroma ingredient and pathological features in gastric cancer[J]. Chin J Dig Surg, 2022, 21( 3): 427- 432. DOI: 10.3760/cma.j.cn115610-20220216-00090.杨彦, 李腾飞, 黄陈. 肿瘤间质成分及病理学特征在胃肿瘤中的研究进展[J]. 中华消化外科杂志, 2022, 21( 3): 427- 432. DOI: 10.3760/cma.j.cn115610-20220216-00090. [10] KWON OS, CHOI SH, KIM JH. Inflammation and hepatic fibrosis, then hepatocellular carcinoma[J]. Korean J Gastroenterol, 2015, 66( 6): 320- 324. DOI: 10.4166/kjg.2015.66.6.320. [11] CHEN H, ZHOU XH, LI JR, et al. Neutrophils: Driving inflammation during the development of hepatocellular carcinoma[J]. Cancer Lett, 2021, 522: 22- 31. DOI: 10.1016/j.canlet.2021.09.011. [12] PETRICK JL, FLORIO AA, ZNAOR A, et al. International trends in hepatocellular carcinoma incidence, 1978–2012[J]. Int J Cancer, 2020, 147( 2): 317- 330. DOI: 10.1002/ijc.32723. [13] TANG A, HALLOUCH O, CHERNYAK V, et al. Epidemiology of hepatocellular carcinoma: Target population for surveillance and diagnosis[J]. Abdom Radiol, 2018, 43( 1): 13- 25. DOI: 10.1007/s00261-017-1209-1. [14] SINGAL AG, LAMPERTICO P, NAHON P. Epidemiology and surveillance for hepatocellular carcinoma: New trends[J]. J Hepatol, 2020, 72( 2): 250- 261. DOI: 10.1016/j.jhep.2019.08.025. [15] YE DY, YANG S, ZHAO LL, et al. Research progress in effects of inflammatory markers on occurrence, development and prognosis of malignant tumors and their mechanisms[J]. J Jilin Univ(Med Ed), 2022, 48( 4): 1079- 1087. DOI: 10.13481/j.1671-587X.20220432.叶德宇, 杨帅, 赵龙龙, 等. 炎性标志物对恶性肿瘤发生发展和预后的影响及其机制的研究进展[J]. 吉林大学学报(医学版), 2022, 48( 4): 1079- 1087. DOI: 10.13481/j.1671-587X.20220432. [16] RICHARDSON JSM, AMINUDIN N, MALEK SN ABD. Chalepin: A compound from Ruta angustifolia L. pers exhibits cell cycle arrest at S phase, suppresses nuclear factor-kappa B(NF-κB) pathway, signal transducer and activation of transcription 3(STAT3) phosphorylation and extrinsic apoptotic pathway in non-small cell lung cancer carcinoma(A549)[J]. Pharmacogn Mag, 2017, 13( Suppl 3): S489-S498. DOI: 10.4103/pm.pm_13_17. [17] VERMA A, SINGH D, ANWAR F, et al. Triterpenoids principle of Wedelia calendulacea attenuated diethynitrosamine-induced hepatocellular carcinoma via down-regulating oxidative stress, inflammation and pathology via NF-kB pathway[J]. Inflammopharmacology, 2018, 26( 1): 133- 146. DOI: 10.1007/s10787-017-0350-3. [18] REN ZR, CHEN Y, SHI L, et al. Sox9/CXCL5 axis facilitates tumour cell growth and invasion in hepatocellular carcinoma[J]. FEBS J, 2022, 289( 12): 3535- 3549. DOI: 10.1111/febs.16357. [19] JIA XQ, WEI SQ, XIONG WJ. CXCL5/NF-κB pathway as a therapeutic target in hepatocellular carcinoma treatment[J]. J Oncol, 2021, 2021: 9919494. DOI: 10.1155/2021/9919494. [20] ZHOU SL, DAI Z, ZHOU ZJ, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils[J]. Carcinogenesis, 2014, 35( 3): 597- 605. DOI: 10.1093/carcin/bgt397. [21] XIA JL, XU XJ, HUANG PX, et al. The potential of CXCL5 as a target for liver cancer-what do we know so far?[J]. Expert Opin Ther Targets, 2015, 19( 2): 141- 146. DOI: 10.1517/14728222.2014.993317. [22] PAN LC, XIAO HY, YIN WJ, et al. Correlation between HSD17B4 expression in rat liver cancer tissues and inflammation or proliferation[J]. Eur Rev Med Pharmacol Sci, 2018, 22( 11): 3386- 3393. DOI: 10.26355/eurrev_201806_15160. [23] YANG YM, KIM SY, SEKI E. Inflammation and liver cancer: Molecular mechanisms and therapeutic targets[J]. Semin Liver Dis, 2019, 39( 1): 26- 42. DOI: 10.1055/s-0038-1676806. [24] XING Y, LIU Y, QI Z, et al. LAGE3 promoted cell proliferation, migration, and invasion and inhibited cell apoptosis of hepatocellular carcinoma by facilitating the JNK and ERK signaling pathway[J]. Cell Mol Biol Lett, 2021, 26( 1): 49. DOI: 10.1186/s11658-021-00295-4. [25] BHOSALE PB, KIM HH, ABUSALIYA A, et al. Structural and functional properties of activator protein-1 in cancer and inflammation[J]. Evid Based Complementary Altern Med, 2022, 2022: 1- 8. DOI: 10.1155/2022/9797929. [26] TAN WL, LUO X, LI WD, et al. TNF-α is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma[J]. EBioMedicine, 2019, 40: 446- 456. DOI: 10.1016/j.ebiom.2018.12.047. [27] DOLCET X, LLOBET D, PALLARES J, et al. NF-kB in development and progression of human cancer[J]. Virchows Arch, 2005, 446( 5): 475- 482. DOI: 10.1007/s00428-005-1264-9. -



 PDF下载 ( 1783 KB)
PDF下载 ( 1783 KB)

 下载:
下载: