缺氧诱导因子1α(HIF-1α)/Yes相关蛋白(YAP)在非酒精性脂肪性肝病中的调控作用
DOI: 10.3969/j.issn.1001-5256.2023.09.024
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:张华负责拟定写作思路并撰写文章;寇萱萱、邓婧鑫参与相关文献及数据的收集与整理;张建刚审校并修改论文。
Regulatory role of hypoxia-inducible factor-1α/Yes-associated protein in nonalcoholic fatty liver disease
-
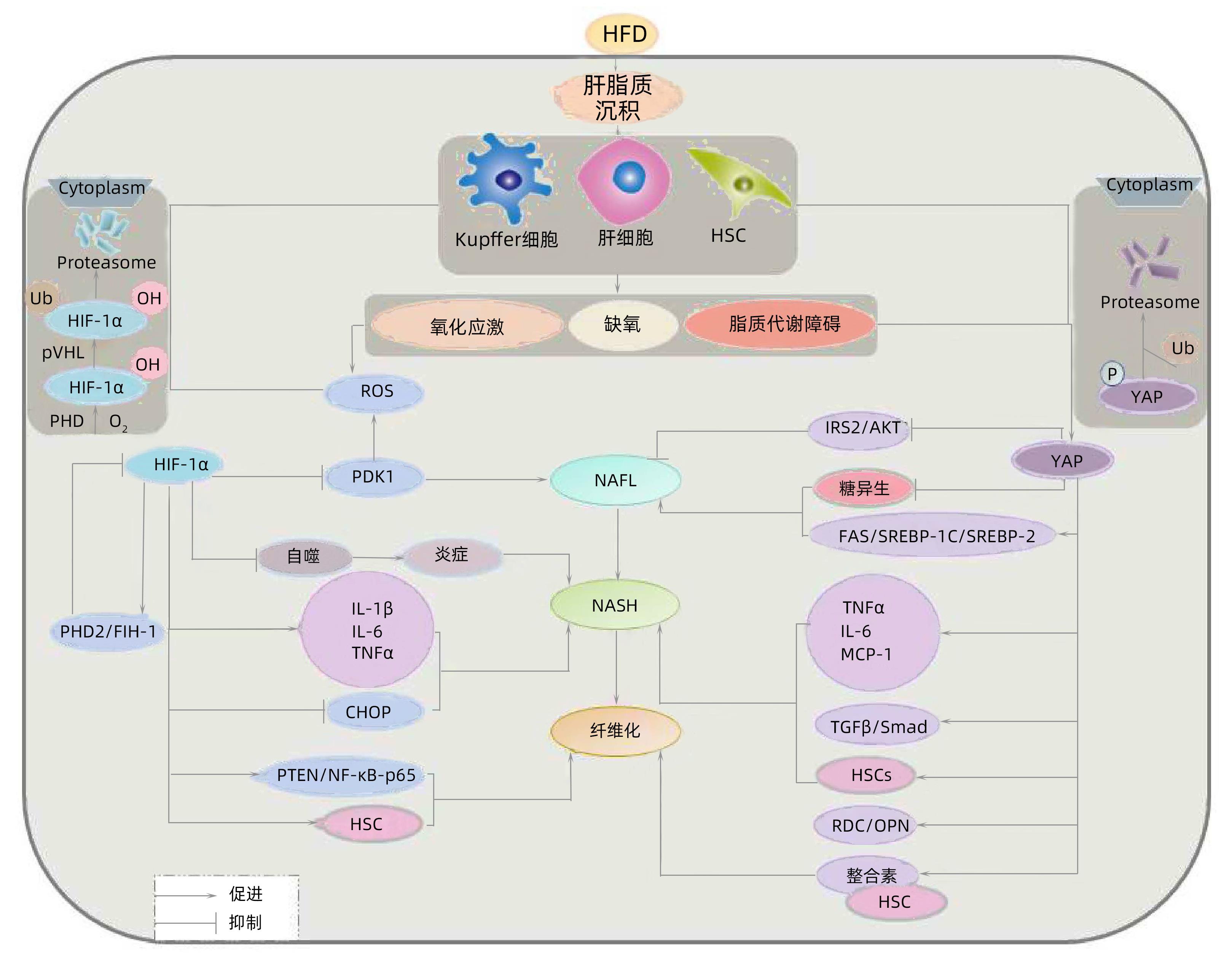
摘要: 非酒精性脂肪性肝病(NAFLD)是目前最为常见的慢性肝病,并与多种代谢性疾病密切相关,如2型糖尿病、胰岛素抵抗,以及与高血压和血脂异常相关的心脑血管并发症。NAFLD病因和病理机制复杂,微环境因素和基因表达调节异常存在于疾病进展的各个阶段,并通过累加效应促进疾病发展。缺氧诱导因子(HIF)是核转录因子、Yes相关蛋白(YAP)是转录辅助调节因子,二者通过调节肝脂质沉积与氧化应激,促进炎性因子释放,与NAFLD进展密切相关。本文对HIF-1α/YAP在NAFLD及其相关代谢性疾病进展中的作用进行综述,为探索NAFLD疾病进展过程中的相关治疗靶点提供理论依据。
-
关键词:
- 非酒精性脂肪性肝病 /
- 代谢综合征 /
- 缺氧诱导因子1, α亚基 /
- Yes相关蛋白
Abstract: Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the world and is closely associated with a variety of metabolic diseases, such as type 2 diabetes, insulin resistance, and cardiovascular and cerebrovascular complications associated with hypertension and dyslipidemia. NAFLD has complex etiologies and pathological mechanisms, and abnormal microenvironmental factors and gene expression regulation exist in all stages of disease progression and promote disease progression through cumulative effects. Hypoxia-inducible factors are hypoxia-inducible transcription factors, and Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif is a transcriptional coactivator, both of which are closely associated with the progression of NAFLD by regulating lipid deposition and oxidative stress in the liver and promoting the release of inflammatory factors. This article reviews the role of hypoxia-inducible factor-1α/YAP in the progression of NAFLD and its related metabolic diseases, so as to provide a theoretical basis for related therapeutic targets in the progression of NAFLD. -
[1] European Association for the Study of the Liver(EASL), European Association for the Study of Diabetes(EASD), European Association for the Study of Obesity(EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease[J]. Obes Facts, 2016, 9( 2): 65- 90. DOI: 10.1159/000443344. [2] ZHOU JH, ZHOU F, WANG WX, et al. Epidemiological features of NAFLD from 1999 to 2018 in China[J]. Hepatology, 2020, 71( 5): 1851- 1864. DOI: 10.1002/hep.31150. [3] ZHU CX, ZHU Q, WANG C, et al. Hostile takeover: Manipulation of HIF-1 signaling in pathogen-associated cancers(review)[J]. Int J Oncol, 2016, 49( 4): 1269- 1276. DOI: 10.3892/ijo.2016.3633. [4] MOYA IM, HALDER G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine[J]. Nat Rev Mol Cell Biol, 2019, 20( 4): 211- 226. DOI: 10.1038/s41580-018-0086-y. [5] POLYZOS SA, KOUNTOURAS J, MANTZOROS CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics[J]. Metabolism, 2019, 92: 82- 97. DOI: 10.1016/j.metabol.2018.11.014. [6] COMINGUEZ DC, PARK YJ, KANG YM, et al. Clitorin ameliorates western diet-induced hepatic steatosis by regulating lipogenesis and fatty acid oxidation in vivo and in vitro[J]. Sci Rep, 2022, 12( 1): 4154. DOI: 10.1038/s41598-022-07937-3. [7] MA B, CHEN Y, CHEN L, et al. Hypoxia regulates hippo signalling through the SIAH2 ubiquitin E3 ligase[J]. Nat Cell Biol, 2015, 17( 1): 95- 103. DOI: 10.1038/ncb3073. [8] HE Y, YANG WH, GAN LL, et al. Silencing HIF-1α aggravates non-alcoholic fatty liver disease in vitro through inhibiting PPAR-α/ANGPTL4 singling pathway[J]. Gastroenterol Hepatol, 2021, 44( 5): 355- 365. DOI: 10.1016/j.gastrohep.2020.09.014. [9] ARAI T, TANAKA M, GODA N. HIF-1-dependent lipin1 induction prevents excessive lipid accumulation in choline-deficient diet-induced fatty liver[J]. Sci Rep, 2018, 8( 1): 14230. DOI: 10.1038/s41598-018-32586-w. [10] KOBAYASHI Y, OGURO A, IMAOKA S. Feedback of hypoxia-inducible factor-1alpha(HIF-1alpha) transcriptional activity via redox factor-1(Ref-1) induction by reactive oxygen species(ROS)[J]. Free Radic Res, 2021, 55( 2): 154- 164. DOI: 10.1080/10715762.2020.1870685. [11] JEONG SH, KIM HB, KIM MC, et al. Hippo-mediated suppression of IRS2/AKT signaling prevents hepatic steatosis and liver cancer[J]. J Clin Invest, 2018, 128( 3): 1010- 1025. DOI: 10.1172/JCI95802. [12] SHU ZP, GAO Y, ZHANG GP, et al. A functional interaction between Hippo-YAP signalling and SREBPs mediates hepatic steatosis in diabetic mice[J]. J Cell Mol Med, 2019, 23( 5): 3616- 3628. DOI: 10.1111/jcmm.14262. [13] HU Y, SHIN DJ, PAN H, et al. YAP suppresses gluconeogenic gene expression through PGC1α[J]. Hepatology, 2017, 66( 6): 2029- 2041. DOI: 10.1002/hep.29373. [14] WONG RJ, CHEUNG R, AHMED A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S[J]. Hepatology, 2014, 59( 6): 2188- 2195. DOI: 10.1002/hep.26986. [15] CAI N, ZHAO X, JING YY, et al. Autophagy protects against palmitate-induced apoptosis in hepatocytes[J]. Cell Biosci, 2014, 4: 28. DOI: 10.1186/2045-3701-4-28. [16] WANG XJ, de CARVALHO RIBEIRO M, IRACHETA-VELLVE A, et al. Macrophage-specific hypoxia-inducible factor-1α contributes to impaired autophagic flux in nonalcoholic steatohepatitis[J]. Hepatology, 2019, 69( 2): 545- 563. DOI: 10.1002/hep.30215. [17] HERNÁNDEZ A, GENG YN, SEPÚLVEDA R, et al. Chemical hypoxia induces pro-inflammatory signals in fat-laden hepatocytes and contributes to cellular crosstalk with Kupffer cells through extracellular vesicles[J]. Biochim Biophys Acta Mol Basis Dis, 2020, 1866( 6): 165753. DOI: 10.1016/j.bbadis.2020.165753. [18] LI T, XIAN WJ, GAO Y, et al. Higd1a protects cells from lipotoxicity under high-fat exposure[J]. Oxid Med Cell Longev, 2019, 2019: 6051262. DOI: 10.1155/2019/6051262. [19] SHIN MK, DRAGER LF, YAO QL, et al. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1α[J]. PLoS One, 2012, 7( 10): e46562. DOI: 10.1371/journal.pone.0046562. [20] LUEDDE T, KAPLOWITZ N, SCHWABE RF. Cell death and cell death responses in liver disease: Mechanisms and clinical relevance[J]. Gastroenterology, 2014, 147( 4): 765- 783. DOI: 10.1053/j.gastro.2014.07.018. [21] PIERANTONELLI I, SVEGLIATI-BARONI G. Nonalcoholic fatty liver disease: Basic pathogenetic mechanisms in the progression from NAFLD to NASH[J]. Transplantation, 2019, 103( 1): e1-e13. DOI: 10.1097/TP.0000000000002480. [22] WREE A, BRODERICK L, CANBAY A, et al. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms[J]. Nat Rev Gastroenterol Hepatol, 2013, 10( 11): 627- 636. DOI: 10.1038/nrgastro.2013.149. [23] YOO W, NOH KH, AHN JH, et al. HIF-1α expression as a protective strategy of HepG2 cells against fatty acid-induced toxicity[J]. J Cell Biochem, 2014, 115( 6): 1147- 1158. DOI: 10.1002/jcb.24757. [24] CHA JY, KIM DH, CHUN KH. The role of hepatic macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis[J]. Lab Anim Res, 2018, 34( 4): 133- 139. DOI: 10.5625/lar.2018.34.4.133. [25] TOSELLO-TRAMPONT AC, LANDES SG, NGUYEN V, et al. Kupffer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production[J]. J Biol Chem, 2012, 287( 48): 40161- 40172. DOI: 10.1074/jbc.M112.417014. [26] ARDESTANI A, LUPSE B, MAEDLER K. Hippo signaling: Key emerging pathway in cellular and whole-body metabolism[J]. Trends Endocrinol Metab, 2018, 29( 7): 492- 509. DOI: 10.1016/j.tem.2018.04.006. [27] SONG K, KWON H, HAN C, et al. Yes-associated protein in kupffer cells enhances the production of proinflammatory cytokines and promotes the development of nonalcoholic steatohepatitis[J]. Hepatology, 2020, 72( 1): 72- 87. DOI: 10.1002/hep.30990. [28] YIMLAMAI D, CHRISTODOULOU C, GALLI GG, et al. Hippo pathway activity influences liver cell fate[J]. Cell, 2014, 157( 6): 1324- 1338. DOI: 10.1016/j.cell.2014.03.060. [29] MOORING M, FOWL BH, LUM SZC, et al. Hepatocyte stress increases expression of yes-associated protein and transcriptional coactivator with PDZ-binding motif in hepatocytes to promote parenchymal inflammation and fibrosis[J]. Hepatology, 2020, 71( 5): 1813- 1830. DOI: 10.1002/hep.30928. [30] CHEN P, LUO QH, HUANG C, et al. Pathogenesis of non-alcoholic fatty liver disease mediated by YAP[J]. Hepatol Int, 2018, 12( 1): 26- 36. DOI: 10.1007/s12072-017-9841-y. [31] WU W, LI WP, WEI JJ, et al. Chronic intermittent hypoxia accelerates liver fibrosis in rats with combined hypoxia and nonalcoholic steatohepatitis via angiogenesis rather than endoplasmic reticulum stress[J]. Acta Biochim Biophys Sin, 2019, 51( 2): 159- 167. DOI: 10.1093/abbs/gmy169. [32] HAN J, HE YP, ZHAO H, et al. Hypoxia inducible factor-1 promotes liver fibrosis in nonalcoholic fatty liver disease by activating PTEN/p65 signaling pathway[J]. J Cell Biochem, 2019, 120( 9): 14735- 14744. DOI: 10.1002/jcb.28734. [33] MESARWI OA, SHIN MK, BEVANS-FONTI S, et al. Hepatocyte hypoxia inducible factor-1 mediates the development of liver fibrosis in a mouse model of nonalcoholic fatty liver disease[J]. PLoS One, 2016, 11( 12): e0168572. DOI: 10.1371/journal.pone.0168572. [34] ZHAN L, HUANG C, MENG XM, et al. Hypoxia-inducible factor-1alpha in hepatic fibrosis: A promising therapeutic target[J]. Biochimie, 2015, 108: 1- 7. DOI: 10.1016/j.biochi.2014.10.013. [35] KAMINSKY-KOLESNIKOV Y, RAUCHBACH E, ABU-HALAKA D, et al. Cholesterol induces nrf-2- and HIF-1α-dependent hepatocyte proliferation and liver regeneration to ameliorate bile acid toxicity in mouse models of NASH and fibrosis[J]. Oxid Med Cell Longev, 2020, 2020: 5393761. DOI: 10.1155/2020/5393761. [36] SALLOUM S, JEYARAJAN AJ, KRUGER AJ, et al. Fatty acids activate the transcriptional coactivator YAP1 to promote liver fibrosis via p38 mitogen-activated protein kinase[J]. Cell Mol Gastroenterol Hepatol, 2021, 12( 4): 1297- 1310. DOI: 10.1016/j.jcmgh.2021.06.003. [37] SONG ZL, CHEN W, ATHAVALE D, et al. Osteopontin takes center stage in chronic liver disease[J]. Hepatology, 2021, 73( 4): 1594- 1608. DOI: 10.1002/hep.31582. [38] MACHADO MV, MICHELOTTI GA, PEREIRA TA, et al. Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease[J]. J Hepatol, 2015, 63( 4): 962- 970. DOI: 10.1016/j.jhep.2015.05.031. [39] MARTIN K, PRITCHETT J, LLEWELLYN J, et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis[J]. Nat Commun, 2016, 7: 12502. DOI: 10.1038/ncomms12502. [40] SHEKA AC, ADEYI O, THOMPSON J, et al. Nonalcoholic steatohepatitis: A review[J]. JAMA, 2020, 323( 12): 1175- 1183. DOI: 10.1001/jama.2020.2298. [41] LI JP, SUN Y, KONG XB, et al. Changes of serum cytokeratin-18 fragment M30,adiponectin and retinol binding protein 4 in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease and their clinical significance[J]. J Clin Exp Med, 2022, 21( 9): 941- 945. DOI: 10.3969/j.issn.1671-4695.2022.09.012.李江佩, 孙音, 孔祥波, 等. 2型糖尿病合并非酒精性脂肪性肝病患者血清细胞角蛋白-18片段M30、脂联素和视黄醇结合蛋白4水平的变化及临床意义[J]. 临床和实验医学杂志, 2022, 21( 9): 941- 945. DOI: 10.3969/j.issn.1671-4695.2022.09.012. [42] WONG VW, WONG GL, WOO J, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease[J]. Clin Gastroenterol Hepatol, 2021, 19( 10): 2161- 2171. DOI: 10.1016/j.cgh.2020.10.046. [43] GONZALEZ FJ, XIE C, JIANG CT. The role of hypoxia-inducible factors in metabolic diseases[J]. Nat Rev Endocrinol, 2018, 15( 1): 21- 32. DOI: 10.1038/s41574-018-0096-z. [44] ANENI EC, ONI ET, MARTIN SS, et al. Blood pressure is associated with the presence and severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk[J]. J Hypertens, 2015, 33( 6): 1207- 1214. DOI: 10.1097/HJH.0000000000000532. [45] BONNET F, GASTALDELLI A, PIHAN-LE BARS F, et al. Gamma-glutamyltransferase, fatty liver index and hepatic insulin resistance are associated with incident hypertension in two longitudinal studies[J]. J Hypertens, 2017, 35( 3): 493- 500. DOI: 10.1097/HJH.0000000000001204. [46] BURGUEÑO AL, GIANOTTI TF, MANSILLA NG, et al. Cardiovascular disease is associated with high-fat-diet-induced liver damage and up-regulation of the hepatic expression of hypoxia-inducible factor 1α in a rat model[J]. Clin Sci, 2013, 124( 1): 53- 63. DOI: 10.1042/CS20120151. [47] ZHANG XM, LAM KSL, YE HY, et al. Adipose tissue-specific inhibition of hypoxia-inducible factor 1{alpha}induces obesity and glucose intolerance by impeding energy expenditure in mice[J]. J Biol Chem, 2010, 285( 43): 32869- 32877. DOI: 10.1074/jbc.M110.135509. [48] ASAI Y, YAMADA T, TSUKITA S, et al. Activation of the hypoxia inducible factor 1α subunit pathway in steatotic liver contributes to formation of cholesterol gallstones[J]. Gastroenterology, 2017, 152( 6): 1521- 1535. DOI: 10.1053/j.gastro.2017.01.001. -



 PDF下载 ( 807 KB)
PDF下载 ( 807 KB)

 下载:
下载:


