黄芪提取物调节IL-6/STAT3信号通路治疗马兜铃酸Ⅰ诱导肝肾损伤小鼠模型的效果观察
DOI: 10.3969/j.issn.1001-5256.2023.08.020
Efficacy of Astragali Radix extract in treatment of a mouse model of aristolochic acid Ⅰ-induced liver and renal injury by regulating the IL-6/STAT3 signaling pathway
-
摘要:
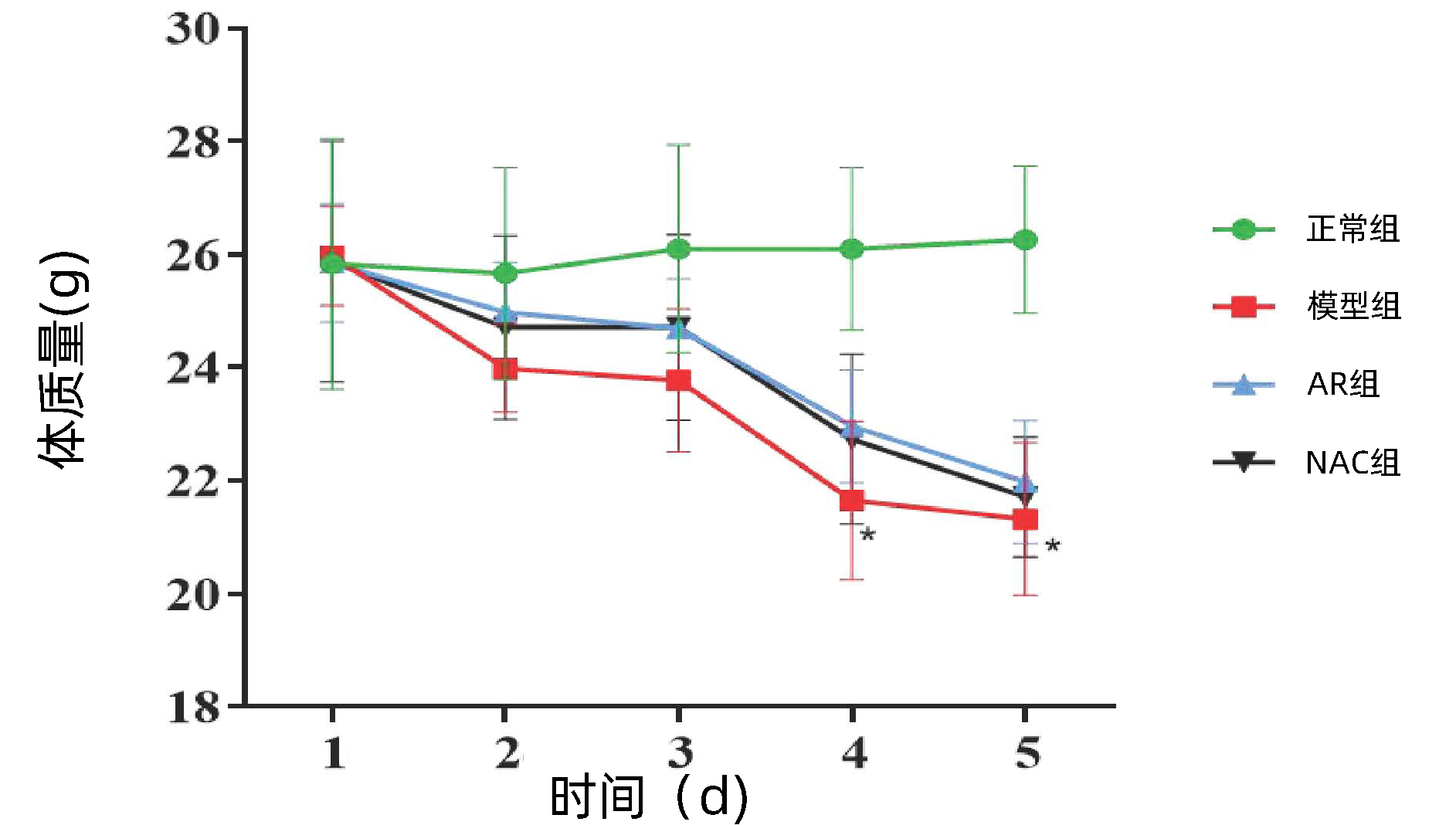
目的 探讨黄芪提取物(AR)改善马兜铃酸Ⅰ(AA Ⅰ)致小鼠急性肝、肾损伤效果及其调控IL-6/STAT3信号通路的作用机制。 方法 健康雄性C57BL/6小鼠38只,采用简单随机分组法分为正常组(n=8)、模型组(n=10)、AR组(n=10)和N-乙酰半胱氨酸(NAC)组(n=10)。模型组小鼠以20 mg/kg AAⅠ腹腔注射,1次/d,持续5 d。正常组小鼠腹腔注射相同容积羧甲基纤维素钠。AR组、NAC组20 mg/kg AAⅠ腹腔注射,1次/d,持续3 d;第4天分别按AR 75 mg/kg、NAC 150 mg/kg小鼠体质量剂量灌胃,1次/d,持续8 d。NAC为阳性对照药。给药造模结束后,处死小鼠并收集血清及肝、肾组织。试剂盒检测血清ALT、AST、肌酐(SCr)、尿素氮(BUN)水平;HE染色观察肝、肾组织病理;荧光PCR及免疫组化分析肝、肾组织中p-STAT3表达量;酶联免疫吸附实验检测肝、肾组织IL-6、IL-1β及TNF-α表达水平。计量资料多组间比较采用单因素方差分析,进一步两组间比较采用SNK-q检验。 结果 与正常组小鼠相比,模型组小鼠肾体比上升(P < 0.05);与模型组相比,AR组ALT、AST、SCr和BUN水平显著降低(F值分别为49.29、31.31、58.89、85.88,P值均 < 0.01);HE染色结果表明,AR可有效减轻AAⅠ导致的肝、肾组织结构破坏和炎性细胞浸润;荧光PCR及免疫组化染色结果表明,AR可减少肝、肾组织p-STAT3表达;酶联免疫吸附检测发现,AR可下调IL-6、IL-1β及TNF-α表达。NAC与AR效应相似,两者间无明显差异。 结论 AR对AAⅠ所致急性肝、肾损伤有保护作用,其部分作用机制可能与抑制IL-6/STAT3信号通路激活,减轻炎症反应有关。 -
关键词:
- 马兜铃酸 /
- 化学性与药物性肝损伤 /
- 急性肾损伤 /
- STAT3转录因子 /
- 黄芪
Abstract:Objective To investigate the mechanism of action of Astragali Radix (AR) extract in improving aristolochic acid Ⅰ (AA Ⅰ)-induced acute liver and renal injury in mice by regulating the IL-6/STAT3 signaling pathway. Methods A total of 38 healthy male C57BL/6 mice were randomly divided into normal group with 8 mice, model group with 10 mice, AR group with 10 mice, and N-Acetyl-L-cysteine (NAC) group with 10 mice. The model group mice were intraperitoneally injected with 20 mg/kg AAⅠ once a day for 5 days. Normal mice were intraperitoneally injected with the same volume of Carboxymethyl cellulose sodium. AR group and NAC group received intraperitoneal injection of 20 mg/kg AAⅠ once a day for 3 days; On the 4th day, mice were gavaged with AR 75 mg/kg and NAC 150 mg/kg body mass doses, once a day, for 8 days. NAC was used as a positive control drug. After the end of administration and modeling, the mice were sacrificed to collect serum samples and liver and renal tissue samples. The kit was used to measure the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (SCr), and blood urea nitrogen (BUN); HE staining was used to observe liver and renal histopathology; quantitative real-time PCR and immunohistochemistry were used to measure the expression level of p-STAT3 in the liver and renal tissue; ELISA was used to measure the expression levels of interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) in the liver and renal tissue. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the SNK-q test was used for further comparison between two groups. Results Compared with the normal group, the model group had a significant increase in kidney-to-body ratio (P < 0.05). Compared with the model group, the AR group had significant reductions in the levels of ALT, AST, SCr, and BUN (F=49.29, 31.31, 58.89, and 85.88, all P < 0.01). HE staining showed that AR could effectively alleviate AAⅠ -induced structural damage and inflammatory cell infiltration in the liver and renal tissue; quantitative real-time PCR and immunohistochemistry showed that AR could reduce the expression of p-STAT3 in the liver and renal tissues; ELISA showed that AR could downregulate the expression of IL-6, IL-1β, and TNF-α. NAC and AR had a similar effect with no significant differences. Conclusion AR exerts a protective effect against AAⅠ-induced acute liver and renal injury, possibly by inhibiting the activation of the IL-6/STAT3 signaling pathway and alleviating inflammatory response. -
表 1 荧光定量PCR基因引物序列
Table 1. qRT-PCR primer sequences of each gene
基因 引物 序列 产物长度(bp) STAT3 上游 5′-TGTCAGATCACATGGGCTAAAT-3′ 88 下游 5′-GGTCGATGATATTGTCTAGCCA-3′ β-actin 上游 5′-TGACGAGGCCCAGAGCAAGA-3′ 330 下游 5′-ATGGGCACAGTGTGGGTGAC-3′ 表 2 AR对AAⅠ小鼠模型脏器指数的影响
Table 2. Effect of AR on organ index of AAⅠ model mice
组别 动物数(只) 肝体比(%) 肾体比(‰) 正常组 8 5.36±0.59 7.56±0.66 模型组 8 5.09±0.27 10.76±1.031) AR组 10 5.26±0.32 9.98±0.991)2) NAC组 9 5.24±0.32 10.68±0.451) F值 0.38 22.63 P值 0.70 < 0.01 注:与正常组相比,1)P < 0.01;与模型组相比,2)P < 0.01。 表 3 AR对AAⅠ模型小鼠肝、肾功能的影响
Table 3. Effects of AR on liver and kidney function of AAⅠ model mice
组别 动物数(只) 肝功能 肾功能 ALT(U/L) AST(U/L) SCr (mmol/L) BUN (mmol/L) 正常组 8 36.50±17.52 79.75±35.63 13.50±1.07 8.78±2.44 模型组 8 124.40±15.621) 453.90±99.911) 288.40±2.391) 109.70±13.521) AR组 10 95.80±16.102) 263.20±92.202) 195.70±48.562) 80.82±19.182) NAC组 9 112.20±13.83 357.80±77.763) 241.30±67.76 79.49±9.542) F值 49.29 31.31 58.89 85.88 P值 < 0.01 < 0.01 < 0.01 < 0.01 注:与正常组相比,1)P < 0.01;与模型组相比,2)P < 0.01,3)P < 0.05。 表 4 各组小鼠肝、肾组织STAT3 mRNA变化
Table 4. Changes of STAT3 mRNA in liver and kidney of mice in each group
组别 动物数(只) 肝STAT3 mRNA 肾STAT3 mRNA 正常组 8 0.63±0.20 0.71±0.09 模型组 8 1.53±0.161) 1.61±0.151) AR组 10 0.97±0.202) 1.15±0.152) NAC组 9 1.02±0.232) 1.18±0.102) F值 29.88 72.40 P值 < 0.01 < 0.01 注:与正常组相比,1)P < 0.01;与模型组相比,2)P < 0.01。 表 5 各组小鼠肝、肾组织中p-STAT3免疫组化结果半定量分析
Table 5. Semi-quantitative analysis of p-STAT3 immunohistochemical results in liver and kidney tissues of mice in each group
组别 动物数(只) 肝脏p-STAT3阳性染色面积比(%) 肾脏p-STAT3阳性染色面积比(%) 正常组 8 0.36±0.08 0.21±0.03 模型组 8 8.62±0.241) 12.70±10.131) AR组 10 4.78±0.132) 6.20±0.162) NAC组 9 6.78±0.122) 9.29±0.102) F值 491.97 2 101.97 P值 < 0.01 < 0.01 注:与正常组相比,1)P < 0.01;与模型组相比,2)P < 0.01。 表 6 AR对AAⅠ模型肝、肾组织中IL-6、IL-1β和TNF-α水平的影响
Table 6. Effects of AR on Levels of IL-6, IL-1β and TNF-α in liver and kidney tissues
组别 动物数(只) 肝组织(pg/mg) 肾组织(pg/mg) IL-6 IL-1β TNF-α IL-6 IL-1β TNF-α 正常组 8 24.52±2.92 57.06±8.91 147.40±17.18 31.00±6.11 42.11±7.78 99.18±32.36 模型组 8 55.70±10.731) 141.50±30.271) 355.90±15.481) 158.60±12.531) 329.80±45.231) 1 051.00±12.531) AR组 10 29.05±3.032) 70.67±13.702) 185.20±19.822) 36.51±23.652) 67.54±28.792) 105.30±31.712) NAC组 9 45.89±13.893) 75.98±9.532) 192.70±9.332) 45.56±9.082) 76.08±17.542) 171.80±31.242) F值 22.05 38.03 270.37 123.21 186.31 2 098.69 P值 < 0.01 < 0.01 < 0.01 < 0.01 < 0.01 < 0.01 注:与正常组相比,1)P < 0.01;与模型组相比,2)P < 0.01,3)P < 0.05。 -
[1] JI HJ, LI JY, WU SF, et al. Two new aristolochic acid analogues from the roots of Aristolochia contorta with significant cytotoxic activity[J]. Molecules, 2020, 26(1): 44. DOI: 10.3390/molecules26010044. [2] HAN JY, XIAN Z, ZHANG YS, et al. Systematic overview of aristolochic acids: Nephrotoxicity, carcinogenicity, and underlying mechanisms[J]. Front Pharmacol, 2019, 10: 648. DOI: 10.3389/fphar.2019.00648. [3] ZHU GR, WANG J, HUANG K, et al. A transcriptomic analysis of acute hepatotoxicity induced by aristolochic acid Ⅰ in mice[J]. J Clin Hepatol, 2021, 37(10): 2389-2394. DOI: 10.3969/j.issn.1001-5256.2021.10.026.朱哿瑞, 王静, 黄恺, 等. 马兜铃酸Ⅰ致小鼠急性肝毒性的转录组学分析[J]. 临床肝胆病杂志, 2021, 37(10): 2389-2394. DOI: 10.3969/j.issn.1001-5256.2021.10.026. [4] WANG F, WANG J, HUANG K, et al. Study on mechanism of aristolochic acid I induced acute kidney injury[J]. Nat Prod Res Dev, 2022, 34(5): 848-855. DOI: 10.16333/j.1001-6880.2022.5.014.王帆, 王静, 黄恺, 等. 马兜铃酸I致急性肾损伤的分子机制研究[J]. 天然产物研究与开发, 2022, 34(5): 848-855. DOI: 10.16333/j.1001-6880.2022.5.014. [5] CAO YX, LI K, QIN XM, et al. Comparative study on different areas of Astragali Radix based on oligosaccharides characteristic map and immunological activity evaluation of partial acid hydrolyzed[J]. Chin Tradit Herb Drugs, 2020, 51(21): 5598-5606. DOI: 10.7501/j.issn.0253-2670.2020.21.025.曹宇欣, 李科, 秦雪梅, 等. 基于部分酸水解寡糖特征图谱及免疫活性评价的不同产地黄芪的品质比较[J]. 中草药, 2020, 51(21): 5598-5606. DOI: 10.7501/j.issn.0253-2670.2020.21.025. [6] HU NN, ZHANG XJ. Research progress on chemical constituents and pharmacological effects of Astragalus membranaceus[J]. Inf Tradit Chin Med, 2021, 38(1): 76-82. DOI: 10.19656/j.cnki.1002-2406.210118.胡妮娜, 张晓娟. 黄芪的化学成分及药理作用研究进展[J]. 中医药信息, 2021, 38(1): 76-82. DOI: 10.19656/j.cnki.1002-2406.210118. [7] CUI Y, LI HS, SONG NN, et al. Metabonomics reveals that aristolochic acid Ⅰ affects β-oxidation of fatty acids, glucose metabolism and the TCA cycle in the mice liver[J]. Chin J Pharmacovigil, 2019, 16(8): 449-466. DOI: 10.19803/j.1672-8629.2019.08.001.崔媛, 李海山, 宋乃宁, 等. 马兜铃酸Ⅰ影响小鼠肝脏脂肪酸β氧化和糖代谢以及TCA循环的代谢组学研究[J]. 中国药物警戒, 2019, 16(8): 449-466. DOI: 10.19803/j.1672-8629.2019.08.001. [8] LIU X, XIAO Y, GAO HC, et al. Metabonomic study of aristolochic acid I-induced acute renal toxicity urine at female and male C57BL/6J mice based on 1H NMR[J]. Chem J Chin Univ, 2010, 31(5): 927-932. https://www.cnki.com.cn/Article/CJFDTOTAL-GDXH201005016.htm刘霞, 肖瑛, 高红昌, 等. 基于1H NMR代谢组学方法分析马兜铃酸I诱导的雌雄小鼠急性肾毒性[J]. 高等学校化学学报, 2010, 31(5): 927-932. https://www.cnki.com.cn/Article/CJFDTOTAL-GDXH201005016.htm [9] PENG Y, ZHU GR, MA YY, et al. Network pharmacology-based prediction and pharmacological validation of effects of Astragali Radix on acetaminophen-induced liver injury[J]. Front Med, 2022, 9: 697644. DOI: 10.3389/fmed.2022.697644. [10] LUO JH, YANG YB. Effect of N-acetylcysteine on oxidative stress in acute kidney injury induced by cisplatin[J]. Chin J Exp Tradit Med Formulae, 2012, 18(19): 170-175. DOI: 10.13422/j.cnki.syfjx.2012.19.052.罗景慧, 杨迎暴. N-乙酰半胱氨酸对顺铂诱导急性肾损伤后肾脏组织氧化应激水平的影响[J]. 中国实验方剂学杂志, 2012, 18(19): 170-175. DOI: 10.13422/j.cnki.syfjx.2012.19.052. [11] WANG J, ZHAO S, REN BH. Protective effect and mechanism of N-acetylcysteine on cisplatin-induced nephrotoxicity[J]. Chin J Immunol, 2020, 36(4): 390-394. DOI: 10.3969/j.issn.1000-484X.2020.04.002.王健, 赵硕, 任博环. N-乙酰半胱氨酸对顺铂导致肾毒性的保护作用及作用机制分析[J]. 中国免疫学杂志, 2020, 36(4): 390-394. DOI: 10.3969/j.issn.1000-484X.2020.04.002. [12] WANG D, QI J, PAN XQ, et al. The antagonistic effect and mechanism of N-acetylcysteine on acrylamide-induced hepatic and renaltoxicity[J]. Chin J Ind Hyg Occup Dis, 2016, 34(1): 13-17.王敦, 齐健, 潘校琦, 等. N-乙酰半胱氨酸拮抗丙烯酰胺的肝肾毒性及机制[J]. 中华劳动卫生职业病杂志, 2016, 34(1): 13-17. [13] WU N, GAO X, YE ZH, et al. Inheritance and innovation of theory of homogeny of liver and kidney for LI Hanmin[J]. Chin Arch Tradit Chin Med, 2018, 36(3): 619-622. DOI: 10.13193/j.issn.1673-7717.2018.03.025.吴娜, 高翔, 叶之华, 等. 李瀚旻教授对"肝肾同源"理论的继承创新[J]. 中华中医药学刊, 2018, 36(3): 619-622. DOI: 10.13193/j.issn.1673-7717.2018.03.025. [14] ANGER EE, YU F, LI J. Aristolochic acid-induced nephrotoxicity: Molecular mechanisms and potential protective approaches[J]. Int J Mol Sci, 2020, 21(3): 1157. DOI: 10.3390/ijms21031157. [15] DAI ZC, WANG XH, PENG RX, et al. Induction of IL-6Rα by ATF3 enhances IL-6 mediated sorafenib and regorafenib resistance in hepatocellular carcinoma[J]. Cancer Lett, 2022, 524: 161-171. DOI: 10.1016/j.canlet.2021.10.024. [16] CLEMENS MM, KENNON-MCGILL S, VAZQUEZ JH, et al. Exogenous phosphatidic acid reduces acetaminophen-induced liver injury in mice by activating hepatic interleukin-6 signaling through inter-organ crosstalk[J]. Acta Pharm Sin B, 2021, 11(12): 3836-3846. DOI: 10.1016/j.apsb.2021.08.024. [17] CHEN W, YUAN H, CAO WM, et al. Blocking interleukin-6 trans-signaling protects against renal fibrosis by suppressing STAT3 activation[J]. Theranostics, 2019, 9(14): 3980-3991. DOI: 10.7150/thno.32352. [18] ZHENG C, HUANG L, LUO W, et al. Inhibition of STAT3 in tubular epithelial cells prevents kidney fibrosis and nephropathy in STZ-induced diabetic mice[J]. Cell Death Dis, 2019, 10(11): 848. DOI: 10.1038/s41419-019-2085-0. [19] SINCLAIR S. Chinese herbs: A clinical review of Astragalus, Ligusticum, and Schizandrae[J]. Altern Med Rev, 1998, 3(5): 338-344. [20] JIANG H, GU SL, ZHANG YT, et al. Research progress on chemical constituents and pharmacological effects of Astragalus membranaceus[J]. J Anhui Univ Chin Med, 2020, 39(5): 93-96. DOI: 10.3969/j.issn.2095-7246.2020.05.022.姜辉, 顾胜龙, 张玉婷, 等. 黄芪化学成分和药理作用研究进展[J]. 安徽中医药大学学报, 2020, 39(5): 93-96. DOI: 10.3969/j.issn.2095-7246.2020.05.022. [21] LI Y, MA W. Analysis on the medication rule of Chinese medicine in the treatment of interstitial pneumonia based on data mining[J]. Chin Med Herald, 2023, 20(14): 146-150. DOI: 10.20047/j.issn1673-7210.2023.14.31.李杨, 马伟. 基于数据挖掘的含有黄芪的中成药配伍规律研究[J]. 中国医药导报, 2023, 20(14): 146-150. DOI: 10.20047/j.issn1673-7210.2023.14.31. [22] HUANG LJ, DENG XL, QIN JF, et al. Effects of Huangqi injection assisted PHGF therapy on liver function and serum sST2 and IL-33 in patients with viral hepatitis[J]. Clin Misdiagn Misther, 2022, 35(4): 21-25, 30. https://www.cnki.com.cn/Article/CJFDTOTAL-LCWZ202204006.htm黄丽静, 邓喜亮, 覃金凤, 等. 黄芪注射液辅助PHGF治疗对病毒性肝炎患者肝功能及血清sST2、IL-33的影响[J]. 临床误诊误治, 2022, 35(4): 21-25, 30. https://www.cnki.com.cn/Article/CJFDTOTAL-LCWZ202204006.htm [23] WANG SM, GENG JB, WANG M, et al. Therapeutic effect of acetylcysteine on drug-induced liver injury[J]. Chin Hepatol, 2017, 22(1): 32-34. DOI: 10.14000/j.cnki.issn.1008-1704.2017.01.011.王寿明, 耿家宝, 王敏, 等. 乙酰半胱氨酸治疗药物性肝损伤疗效观察[J]. 肝脏, 2017, 22(1): 32-34. DOI: 10.14000/j.cnki.issn.1008-1704.2017.01.011. [24] AI G, WANG M, ZHU JL, et al. Preliminary study on the clinical efficacy of N-acetylcysteine in the treatment of patients with chronic icteric hepatitis B[J]. J Pract Hepatol, 2020, 23(3): 336-339. DOI: 10.3969/j.issn.1672-5069.2020.03.009.艾国, 王鸣, 朱纪玲, 等. 应用N-乙酰半胱氨酸治疗慢性乙型肝炎重度患者临床疗效初步研究[J]. 实用肝脏病杂志, 2020, 23(3): 336-339. DOI: 10.3969/j.issn.1672-5069.2020.03.009. [25] MAGNER K, ILIN JV, CLARK EG, et al. Meta-analytic techniques to assess the association between N-acetylcysteine and acute kidney injury after contrast administration: A systematic review and meta-analysis[J]. JAMA Netw Open, 2022, 5(7): e2220671. DOI: 10.1001/jamanetworkopen.2022.20671. [26] LI QW, LIAO JZ, CHEN WJ, et al. NAC alleviative ferroptosis in diabetic nephropathy via maintaining mitochondrial redox homeostasis through activating SIRT3-SOD2/Gpx4 pathway[J]. Free Radic Biol Med, 2022, 187: 158-170. DOI: 10.1016/j.freeradbiomed.2022.05.024. -



 PDF下载 ( 2922 KB)
PDF下载 ( 2922 KB)

 下载:
下载:





