蒙药蓝盆花抑制肝星状细胞增殖的作用及机制初探
DOI: 10.3969/j.issn.1001-5256.2023.06.015
Role and mechanism of action of the Mongolian medicine Scabiosa atropurea in inhibiting the proliferation of hepatic stellate cells
-
摘要:
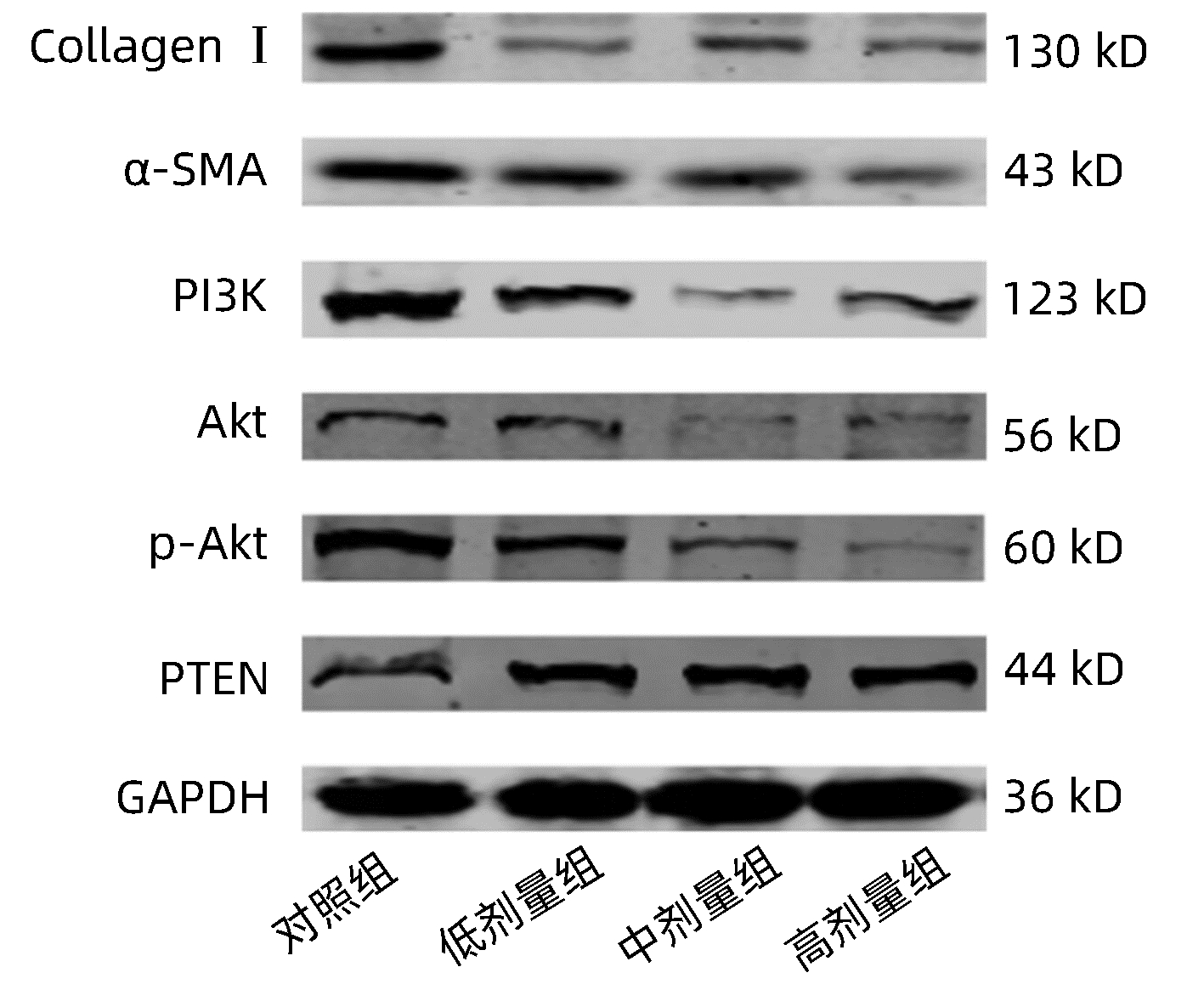
目的 利用细胞实验探究蓝盆花对肝星状细胞增殖的作用及机制。 方法 取20只Wistar大鼠随机分为对照组和给药组,每组10只,对照组以生理盐水灌胃,给药组予蓝盆花灌胃制备含药血清。分别加入对照组血清(10%)、蓝盆花含药血清低剂量(10%)、蓝盆花含药血清中剂量(15%)及蓝盆花含药血清高剂量(20%),用以孵育HSC-T6细胞。MTT法检测不同药物浓度在不同时间段对细胞的影响;流式细胞术检测细胞凋亡情况;qRT-PCR及Western blot检测HSC细胞中纤维化标志物(α-SMA、Collagen Ⅰ)及PI3K/Akt信号通路相关因子mRNA、蛋白表达情况。多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与对照组比较,蓝盆花含药血清低、中、高剂量组细胞OD值均显著降低(P值均<0.05),细胞总凋亡率均显著增高(P值均<0.05)。qRT-PCR结果显示,与对照组比较,蓝盆花含药血清低、中、高剂量组α-SMA、Collagen Ⅰ、PI3K、Akt mRNA表达显著下调,PTEN mRNA表达显著增高(P值均<0.05);Western blot结果显示,与对照组比较,蓝盆花含药血清低、中、高剂量组α-SMA、Collagen Ⅰ、PI3K、Akt、p-Akt蛋白表达显著下调,PTEN蛋白表达显著增高(P值均<0.05)。 结论 蒙药蓝盆花可抑制HSC-T6细胞增殖且促进其凋亡,其机制可能是通过调控纤维化标志物和PI3K/Akt信号通路来发挥抗肝纤维化作用。 Abstract:Objective To investigate the role and mechanism of action of Scabiosa atropurea in inhibiting the proliferation of hepatic stellate cells using cell experiment. Methods A total of 20 Wistar rats were randomly divided into control group and administration group, with 10 rats in each group. The rats in the control group were given normal saline by gavage, and those in the administration group were given Scabiosa atropurea by gavage to prepare drug-containing serum. HSC-T6 cells were incubated with the serum from the control group (10%) or the low-, middle-, and high-dose serum containing Scabiosa atropurea (10%, 15%, and 20%, respectively). MTT assay was used to observe the effect of different drug concentrations on cells in different periods of time; flow cytometry was used to measure cell apoptosis; qRT-PCR and Western blot were used to measure the mRNA and protein expression levels of fibrosis markers (α-SMA, collagen Ⅰ) and PI3K/Akt signaling pathway-related factors in hepatic stellate cells (HSCs). A one-way analysis of variance was used for comparison between multiple groups, and the least significant difference t- test was used for further comparison between two groups. Results Compared with the control group, the low-, middle-, and high-dose serum containing Scabiosa atropurea groups had a significant reduction in the OD value of cells (all P < 0.05) and a significant increase in the overall apoptosis rate of cells (all P < 0.05). The results of qRT-PCR showed that compared with the control group, the low-, middle-, and high-dose serum containing Scabiosa atropurea groups had significant reductions in the mRNA expression levels of α-SMA, collagen Ⅰ, PI3K, and Akt and a significant increase in the mRNA expression level of PTEN (all P < 0.05); Western blot showed that compared with the control group, the low-, middle-, and high-dose serum containing Scabiosa atropurea groups had significant reductions in the protein expression levels of α-SMA, collagen Ⅰ, PI3K, Akt, and p-Akt and a significant increase in the protein expression level of PTEN (all P < 0.05). Conclusion The Mongolian medicine Scabiosa atropurea can inhibit the proliferation of HSC-T6 cells and promote their apoptosis, possibly by regulating fibrosis markers and the PI3K/Akt signaling pathway to exert an anti-liver fibrosis effect. -
Key words:
- Hepatic Fibrosis /
- Hepatic Stellate Cells /
- Scabiosa Atropurea /
- Signal Transduction
-
表 1 各组细胞OD值的检测结果
Table 1. Results of OD value of cells in each group
组别 给药24 h 给药48 h 给药72 h 对照组 0.98±0.01 0.96±0.01 0.98±0.01 低剂量组 0.63±0.042) 0.71±0.042) 0.88±0.021) 中剂量组 0.71±0.042) 0.76±0.012) 0.85±0.031) 高剂量组 0.38±0.052) 0.67±0.072) 0.81±0.032) F值 37.82 20.64 45.07 P值 <0.001 <0.001 <0.001 注:与对照组比较,1)P<0.05, 2)P<0.01。 表 2 各组细胞凋亡率的检测结果
Table 2. Detection results of apoptosis rate in each group
组别 晚期凋亡率(%) 早期凋亡率(%) 总凋亡率(%) 对照组 7.45±0.40 4.48±0.83 11.92±1.08 低剂量组 8.19±1.092) 16.51±0.852) 24.70±1.841) 中剂量组 6.58±0.442) 10.87±0.922) 17.46±0.951) 高剂量组 6.92±0.622) 12.36±0.882) 19.28±1.442) F值 24.36 16.03 20.58 P值 <0.001 <0.001 <0.001 注:与对照组比较,1)P<0.05, 2)P<0.01。 表 3 HSC-T6细胞中α-SMA、Collagen Ⅰ、PI3K、Akt及PTEN的mRNA水平
Table 3. The mRNA expression levels of α-SMA, Collagen Ⅰ, PI3K, Akt and PTEN in HSC-T6 cells
组别 Collagen Ⅰ α-SMA PI3K Akt PTEN 对照组 1.000±0.000 1.000±0.000 1.000±0.000 1.000±0.000 1.000±0.000 低剂量组 0.740±0.0741) 0.697±0.0792) 0.119±0.0142) 0.417±0.1402) 4.820±0.8572) 中剂量组 0.334±0.1282) 0.422±0.0082) 0.274±0.0532) 0.399±0.0992) 2.856±0.0801) 高剂量组 0.284±0.1092) 0.301±0.0202) 0.170±0.0562) 0.341±0.0802) 5.848±0.7742) F值 41.74 62.62 269.90 32.15 41.29 P值 <0.001 <0.001 <0.001 <0.001 <0.001 注:与对照组比较,1)P<0.05, 2)P<0.01。 表 4 HSC-T6细胞中α-SMA、Collagen Ⅰ、PI3K、Akt、p-Akt及PTEN的蛋白表达水平
Table 4. The protein expression levels of α-SMA, Collagen Ⅰ, PI3K, Akt, p-Akt and PTEN in HSC-T6 cells
组别 Collagen Ⅰ α-SMA PI3K Akt p-Akt PTEN 对照组 0.081±0.011 0.346±0.087 0.095±0.013 0.840±0.053 0.632±0.043 1.005±0.005 低剂量组 0.044±0.0052) 0.176±0.0322) 0.038±0.0032) 0.613±0.0202) 0.291±0.0692) 2.779±0.3022) 中剂量组 0.052±0.0052) 0.196±0.0191) 0.049±0.0012) 0.492±0.0512) 0.356±0.0572) 2.889±0.0142) 高剂量组 0.031±0.0052) 0.151±0.0132) 0.045±0.0022) 0.511±0.0562) 0.229±0.0212) 3.413±0.0542) F值 27.86 10.28 44.10 34.36 36.74 139.80 P值 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 注:与对照组比较,1)P<0.05, 2)P<0.01。 -
[1] ZHANG CY, YUAN WG, HE P, et al. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets[J]. World J Gastroenterol, 2016, 22(48): 10512-10522. DOI: 10.3748/wjg.v22.i48.10512. [2] TRAUTWEIN C, FRIEDMAN SL, SCHUPPAN D, et al. Hepatic fibrosis: Concept to treatment[J]. J Hepatol, 2015, 62(1 Suppl): S15-S24. DOI: 10.1016/j.jhep.2015.02.039. [3] HIGASHI T, FRIEDMAN SL, HOSHIDA Y. Hepatic stellate cells as key target in liver fibrosis[J]. Adv Drug Deliv Rev, 2017, 121: 27-42. DOI: 10.1016/j.addr.2017.05.007. [4] ZHANG M, SERNA-SALAS S, DAMBA T, et al. Hepatic stellate cell senescence in liver fibrosis: Characteristics, mechanisms and perspectives[J]. Mech Ageing Dev, 2021, 199: 111572. DOI: 10.1016/j.mad.2021.111572. [5] EZHILARASAN D, SOKAL E, NAJIMI M. Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets[J]. Hepatobiliary Pancreat Dis Int, 2018, 17(3): 192-197. DOI: 10.1016/j.hbpd.2018.04.003. [6] AYD1N MM, AKÇAL1 KC. Liver fibrosis[J]. Turk J Gastroenterol, 2018, 29(1): 14-21. DOI: 10.5152/tjg.2018.17330. [7] SCHUPPAN D, ASHFAQ-KHAN M, YANG AT, et al. Liver fibrosis: Direct antifibrotic agents and targeted therapies[J]. Matrix Biol, 2018, 68-69: 435-451. DOI: 10.1016/j.matbio.2018.04.006. [8] YANG HX, BAI YF, CHANG L, et al. Research progress on the resources and utilization of the Mongolian medicinal plant Cyperus genus[J]. Northwest Pharm J, 2020, 35(5): 779-784. DOI: 10.3969/j.issn.1004-2407.2020.05.031.杨宏昕, 白音夫, 常亮, 等. 蒙药材蓝盆花属植物资源及利用的研究进展[J]. 西北药学杂志, 2020, 35(5): 779-784. DOI: 10.3969/j.issn.1004-2407.2020.05.031. [9] LU C, LI Y, CUI Y, et al. Isolation and functional analysis of genes involved in polyacylated anthocyanin biosynthesis in Blue Senecio cruentus[J]. Front Plant Sci, 2021, 12: 640746. DOI: 10.3389/fpls.2021.640746. [10] JAMES ANTONY JJ, ZAKARIA S, ZAKARIA R, et al. Biochemical analyses of Dendrobium Sabin Blue PLBs during cryopreservation by vitrification[J]. Physiol Mol Biol Plants, 2019, 25(6): 1457-1467. DOI: 10.1007/s12298-019-00703-2. [11] ZHANG CY, YAN YX, GAO XY, et al. Mechanism of anti- hepatic fibrosis action of the monk's medicine seven flavors liver clearing powder: based on UHPLC-TOF-MS and network pharmacology methods[J]. J South Med Univ, 2021, 41(8): 1131-1141. DOI: 10.12122/j.issn.1673-4254.2021.08.02.张春艳, 颜羽昕, 高晓阳, 等. 蒙药七味清肝散抗肝纤维化的作用机制: 基于UHPLC-TOF-MS和网络药理学方法[J]. 南方医科大学学报, 2021, 41(8): 1131-1141. DOI: 10.12122/j.issn.1673-4254.2021.08.02. [12] LIANG J, MENG GSLM, YAN YX, et al. Study on the anti-hepatic fibrosis effect and mechanism of qiwei qinggan powder based on proteomics[J]. China Pharm, 2020, 31(11): 1294-1302. DOI: 10.6039/j.issn.1001-0408.2020.11.03.梁洁, 孟根斯立木, 颜羽昕, 等. 基于蛋白质组学研究七味清肝散的抗肝纤维化作用及机制研究[J]. 中国药房, 2020, 31(11): 1294-1302. DOI: 10.6039/j.issn.1001-0408.2020.11.03. [13] ZHANG CY, JIN R, YAN YX, et al. Exploring the mechanism of action of Bluebonnets against liver fibrosis based on pharmacodynamic and network pharmacological approaches[J]. China J Chin Mater Med, 2022, 47(13): 3609-3618. DOI: 10.19540/j.cnki.cjcmm.20220207.702.张春艳, 金蓉, 颜羽昕, 等. 基于药效学和网络药理方法探讨蓝盆花抗肝纤维化的作用机制[J]. 中国中药杂志, 2022, 47(13): 3609-3618. DOI: 10.19540/j.cnki.cjcmm.20220207.702. [14] LUBECKA K, FLOWER K, BEETCH M, et al. Loci-specific differences in blood DNA methylation in HBV-negative populations at risk for hepatocellular carcinoma development[J]. Epigenetics, 2018, 13(6): 605-626. DOI: 10.1080/15592294.2018.1481706. [15] TAO Y, WANG N, QIU T, et al. The role of autophagy and NLRP3 inflammasome in liver fibrosis[J]. Biomed Res Int, 2020, 2020: 7269150. DOI: 10.1155/2020/7269150. [16] ZHANG M, ZANG S. T cells in fibrosis and fibrotic diseases[J]. Front Immunol, 2020, 11: 1142. DOI: 10.3389/fimmu.2020.01142. [17] KOSTALLARI E, HIRSOVA P, PRASNICKA A, et al. Hepatic stellate cell-derived platelet-derived growth factor receptor-alpha-enriched extracellular vesicles promote liver fibrosis in mice through SHP2[J]. Hepatology, 2018, 68(1): 333-348. DOI: 10.1002/hep.29803. [18] MATSUDA M, SEKI E. Hepatic stellate cell-macrophage crosstalk in liver fibrosis and carcinogenesis[J]. Semin Liver Dis, 2020, 40(3): 307-320. DOI: 10.1055/s-0040-1708876. [19] KHOMICH O, IVANOV AV, BARTOSCH B. Metabolic hallmarks of hepatic stellate cells in liver fibrosis[J]. Cells, 2019, 9(1): 24. DOI: 10.3390/cells9010024. [20] SHI Z, ZHANG K, CHEN T, et al. Transcriptional factor ATF3 promotes liver fibrosis via activating hepatic stellate cells[J]. Cell Death Dis, 2020, 11(12): 1066. DOI: 10.1038/s41419-020-03271-6. [21] KAMM DR, MCCOMMIS KS. Hepatic stellate cells in physiology and pathology[J]. J Physiol, 2022, 600(8): 1825-1837. DOI: 10.1113/JP281061. [22] WANG R, SONG F, LI S, et al. Salvianolic acid A attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways[J]. Drug Des Devel Ther, 2019, 13: 1889-1900. DOI: 10.2147/DDDT.S194787. [23] WU H, CHEN G, WANG J, et al. TIM-4 interference in Kupffer cells against CCL4-induced liver fibrosis by mediating Akt1/Mitophagy signalling pathway[J]. Cell Prolif, 2020, 53(1): e12731. DOI: 10.1111/cpr.12731. [24] XIE Y, SHI X, SHENG K, et al. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review)[J]. Mol Med Rep, 2019, 19(2): 783-791. DOI: 10.3892/mmr.2018.9713. [25] PAPA A, PANDOLFI PP. The PTEN-PI3K axis in cancer[J]. Biomolecules, 2019, 9(4): 153. DOI: 10.3390/biom9040153. [26] XIU AY, DING Q, LI Z, et al. Doxazosin attenuates liver fibrosis by inhibiting autophagy in hepatic stellate cells via activation of the PI3K/Akt/mTOR signaling pathway[J]. Drug Des Devel Ther, 2021, 15: 3643-3659. DOI: 10.2147/DDDT.S317701. [27] LIU X, LIU W, DING C, et al. Taxifolin, extracted from waste larix olgensis roots, attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR and TGF-β1/Smads signaling pathways[J]. Drug Des Devel Ther, 2021, 15: 871-887. DOI: 10.2147/DDDT.S281369. -



 PDF下载 ( 2342 KB)
PDF下载 ( 2342 KB)


 下载:
下载: