叉头转录因子A2、J2在肝细胞癌组织中的表达及意义
DOI: 10.3969/j.issn.1001-5256.2021.06.025
Expression and clinical significance of forkhead box A2 and forkhead box J2 in hepatocellular carcinoma
-
摘要:
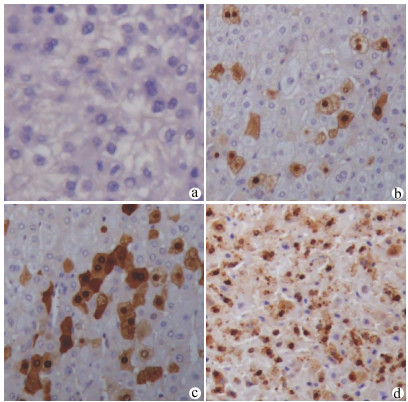
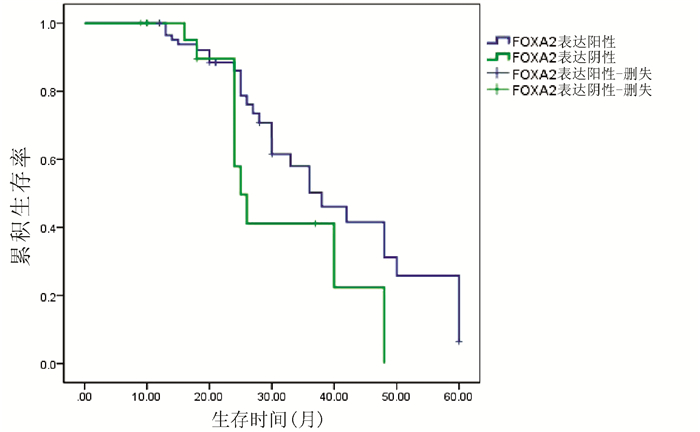
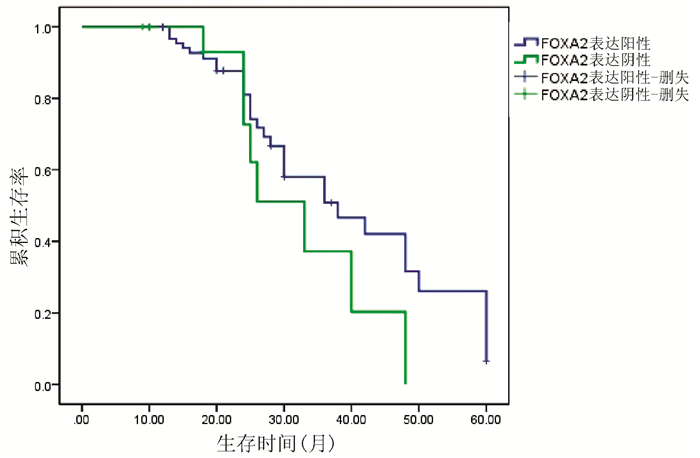
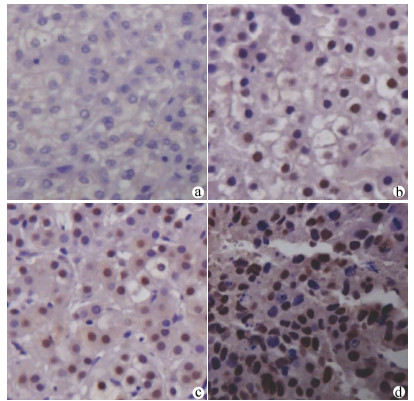
目的 检测肝细胞癌(HCC)组织中叉头(FOX)转录因子A2(FOXA2)与FOXJ2的表达水平,探讨FOXA2、FOXJ2与HCC的相关性。 方法 选取2014年1月—2019年7月于河南中医药大学第一附属医院病理学检查明确为HCC患者54例,收集临床资料和病理组织。采用免疫组化S-P法检测HCC组织中FOXA2与FOXJ2的蛋白水平表达,分析其与HCC相关临床病理特征及患者预后的关系。计数资料比较采用χ2检验与校正χ2检验,FOXJ2与FOXA2表达相关性采用Spearman相关分析法;采用Kaplan-Merier法进行生存期分析;采用Image-Pro Plus进行FOXA2、FOXJ2表达的半定量分析;采用Wilcoxon秩和检验比较组间差异。 结果 HCC组织中FOXA2和FOXJ2蛋白表达阳性率分别为70.37%(38/54)和75.92%(41/54);FOXA2和FOXJ2表达水平间呈显著正相关(rs=0.648,P<0.001)。FOXA2阴性组与阳性组相比,肿瘤直径、分化程度、肿瘤个数、AFP水平差异均有统计学意义(χ2值分别为5.440、4.113、4.352、3.865,P值分别为0.020、0.043、0.037、0.049);FOXJ2阴性组与阳性组比较,分化程度差异有统计学意义(χ2=9.267,P=0.002)。FOXA2、FOXJ2阳性表达组HCC患者的累积生存率均显著高于FOXA2、FOXJ2阴性表达组HCC患者(P值均<0.01)。 结论 FOXA2与FOXJ2的表达水平可能与HCC的发生发展及预后相关,二者在HCC的发生发展中起协同作用。 Abstract:Objective To investigate the expression levels of forkhead box A2 (FOXA2) and forkhead box J2 (FOXJ2) in hepatocellular carcinoma (HCC) tissue and the association of FOXA2 and FOXJ2 with HCC. Methods Clinical data and pathological tissue samples were collected from 54 patients with pathologically confirmed HCC in The First Affiliated Hospital of Henan University of Traditional Chinese Medicine from January 2014 to July 2019. The immunohistochemical SP method was used to measure the protein expression levels of FOXA2 and FOXJ2 in HCC tissue, and their association with HCC-related clinicopathological features and patient prognosis was analyzed. The chi-square test and the adjusted chi-square test were used for comparison of categorical data; a Spearman correlation analysis was performed to investigate the correlation between the expression of FOXA2 and FOXJ2; the Kaplan-Meier method was used for survival analysis; Image-Pro Plus was used to perform the semi-quantitative analysis of the expression of FOXA2 and FOXJ2; the Wilcoxon rank-sum test was used for comparison between groups. Results The positive rates of the protein expression of FOXA2 and FOXJ2 in HCC tissue were 70.37% (38/54) and 75.92% (41/54), respectively, and there was a significant positive correlation between the expression levels of FOXA2 and FOXJ2 (rs=0.648, P < 0.001). In both negative and positive groups, the expression level of FOXA2 was associated with tumor diameter, degree of tumor differentiation, number of tumors, and alpha-fetoprotein (χ2=5.440, 4.113, 4.352, and 3.865, P=0.020, 0.043, 0.037, and 0.049), and the expression level of FOXJ2 was associated with the degree of tumor differentiation (χ2=9.267, P=0.002). The group with positive expression of FOXA2 and FOXJ2 had a significantly higher cumulative survival rate than the group with negative expression of FOXA2 and FOXJ2 (P < 0.01). Conclusion The expression levels of FOXA2 and FOXJ2 are associated with the development, progression, and prognosis of HCC, and they have a synergistic effect in the development and progression of HCC. -
Key words:
- Carcinoma, Hepatocellular /
- Forkhead Transcription Factors /
- Prognosis
-
表 1 FOXA2、FOXJ2表达的半定量分析
项目 FOXA2 FOXJ2 Area IOD Area IOD 不表达 24.60±13.35 3.51±1.91 24.60±13.35 2.96±1.63 低表达 204.53±147.05 84.15±70.3 175.46±537.81 13.14±110.56 中度表达 310.03±214.34 89.02±69.06 400.69±1 551.80 180.63±695.20 高表达 577.11±1212.88 445.26±939.64 1 505.42±9 001.71 652.10±3 987.02 表 2 FOXA2、FOXJ2的表达与肝癌相关临床病理特征的关系
项目 例数 FOXA2 χ2值 P值 FOXJ2 χ2值 P值 阴性(n=16) 阳性(n=38) 阴性(n=13) 阳性(n=41) 性别(例) 0 1.000 0.006 0.940 男 44 13 31 10 34 女 10 3 7 3 7 年龄(例) 0.558 0.455 0.001 0.971 <60岁 33 11 22 8 25 ≥60岁 21 5 16 5 16 肿瘤直径(例) 5.440 0.020 2.026 0.155 ≤5 cm 30 5 25 5 25 >5 cm 24 11 13 8 16 分化程度(例) 4.113 0.043 9.267 0.002 低/中分化 11 6 5 7 4 高分化 43 10 33 6 37 TNM分期(例) 0.032 0.859 0.453 0.501 Ⅰ~Ⅱ期 43 12 31 9 34 Ⅲ~Ⅳ期 11 4 7 4 7 门静脉癌栓(例) 1.072 0.300 2.612 0.106 有 12 5 7 5 7 无 42 11 31 8 34 转移(例) 0.012 0.913 0 1.000 有 8 3 5 2 6 无 46 13 33 11 35 肿瘤个数(例) 4.352 0.037 1.621 0.203 单个 27 4 23 4 23 多个 27 12 15 9 18 AFP(例) 3.865 0.049 2.038 0.153 ≤20 ng/ml 28 5 23 4 24 >20 ng/ml 26 11 15 9 17 -
[1] Bureau of Medical AdministrationNational Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [2] CHEN W, ZHENG R, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin. 2016, 66(2): 115-32. DOI: 10.3322/caac.21338. [3] SIA D, VILLANUEVA A, FRIEDMAN SL, et al. Liver cancer cell of origin, molecular class, and effects on patient prognosis[J]. Gastroenterology, 2017, 152(4): 745-761. DOI: 10.1053/j.gastro.2016.11.048. [4] HAN JH, KIM DG, NA GH, et al. Evaluation of prognostic factors on recurrence after curative resections for hepatocellular carcinoma[J]. World J Gastroenterol, 2014, 20(45): 17132-17140. DOI: 10.3748/wjg.v20.i45.17132. [5] BOULIN M, DELHOM E, PIERREDON-FOULONGNE MA, et al. Transarterial chemoembolization for hepatocellular carcinoma: An old method, now flavor of the day[J]. Diagn Interv Imaging, 2015, 96(6): 607-615. DOI: 10.1016/j.diii.2015.04.005. [6] YANG J, YAN L, WANG W. Current status of multimodal & combination therapy for hepatocellular carcinoma[J]. Indian J Med Res, 2012, 136(3): 391-403. [7] WANG J, LI W, ZHAO Y, et al. Members of FOX family could be drug targets of cancers[J]. Pharmacol Ther, 2018, 181: 183-196. DOI: 10.1016/j.pharmthera.2017.08.003. [8] KATOH M, IGARASHI M, FUKUDA H, et al. Cancer genetics and genomics of human FOX family genes[J]. Cancer Lett, 2013, 328(2): 198-206. DOI: 10.1016/j.canlet.2012.09.017. [9] KATOH M, KATOH M. Human FOX gene family (Review)[J]. Int J Oncol, 2004, 25(5): 1495-1500. http://europepmc.org/abstract/MED/15492844 [10] MYATT SS, LAM EW. The emerging roles of forkhead box (Fox) proteins in cancer[J]. Nat Rev Cancer, 2007, 7(11): 847-859. DOI: 10.1038/nrc2223. [11] LIU J, YU Z, XIAO Y, et al. Coordination of FOXA2 and SIRT6 suppresses the hepatocellular carcinoma progression through ZEB2 inhibition[J]. Cancer Manag Res, 2018, 10: 391-402. DOI: 10.2147/CMAR.S150552. [12] ZHANG H, TANG QF, SUN MY, et al. ARHGAP9 suppresses the migration and invasion of hepatocellular carcinoma cells through up-regulating FOXJ2/E-cadherin[J]. Cell Death Dis, 2018, 9(9): 916. DOI: 10.1038/s41419-018-0976-0. [13] SHAN Y, CHANG T, SHI S, et al. Foxj2 overexpression is associated with poor prognosis, progression, and metastasis in nasopharyngeal carcinoma[J]. Onco Targets Ther, 2017, 10: 3733-3741. DOI: 10.2147/OTT.S134915. [14] TSUCHIYA N, SAWADA Y, ENDO I, et al. Biomarkers for the early diagnosis of hepatocellular carcinoma[J]. World J Gastroenterol, 2015, 21(37): 10573-10583. DOI: 10.3748/wjg.v21.i37.10573. [15] LI H, CHEN D, ZHANG J. Analysis of intron sequence features associated with transcriptional regulation in human genes[J]. PLoS One, 2012, 7(10): e46784. DOI: 10.1371/journal.pone.0046784. [16] LEE TI, YOUNG RA. Transcriptional regulation and its misregulation in disease[J]. Cell, 2013, 152(6): 1237-1251. DOI: 10.1016/j.cell.2013.02.014. [17] LEHMANN OJ, SOWDEN JC, CARLSSON P, et al. Fox's in development and disease[J]. Trends Genet, 2003, 19(6): 339-344. DOI: 10.1016/S0168-9525(03)00111-2. [18] HANNENHALLI S, KAESTNER KH. The evolution of Fox genes and their role in development and disease[J]. Nat Rev Genet, 2009, 10(4): 233-240. DOI: 10.1038/nrg2523. [19] JIN Y, LIANG Z, LOU H. The emerging roles of fox family transcription factors in chromosome replication, organization, and genome stability[J]. Cells, 2020, 9(1): 258. DOI: 10.3390/cells9010258. [20] BENAYOUN BA, CABURET S, VEITIA RA. Forkhead transcription factors: Key players in health and disease[J]. Trends Genet, 2011, 27(6): 224-232. DOI: 10.1016/j.tig.2011.03.003. [21] LI J, DANTAS MACHADO AC, GUO M, et al. Structure of the forkhead domain of FOXA2 bound to a complete DNA consensus site[J]. Biochemistry, 2017, 56(29): 3745-3753. DOI: 10.1021/acs.biochem.7b00211. [22] LIU J, YU Z, XIAO Y, et al. Coordination of FOXA2 and SIRT6 suppresses the hepatocellular carcinoma progression through ZEB2 inhibition[J]. Cancer Manag Res, 2018, 10: 391-402. DOI: 10.2147/CMAR.S150552. [23] LI Z, TUTEJA G, SCHUG J, et al. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer[J]. Cell, 2012, 148(1-2): 72-83. DOI: 10.1016/j.cell.2011.11.026. [24] WANG W, YAO LJ, SHEN W, et al. FOXA2 alleviates CCl4-induced liver fibrosis by protecting hepatocytes in mice[J]. Sci Rep, 2017, 7(1): 15532. DOI: 10.1038/s41598-017-15831-6. [25] YAMAMURA N, FUGO K, KISHIMOTO T. Forkhead box protein A2, a pioneer factor for hepatogenesis, is involved in the expression of hepatic phenotype of alpha-fetoprotein-producing adenocarcinoma[J]. Pathol Res Pract, 2017, 213(9): 1082-1088. DOI: 10.1016/j.prp.2017.07.024. [26] QI J, NAKAYAMA K, CARDIFF RD, et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors[J]. Cancer Cell, 2010, 18(1): 23-38. DOI: 10.1016/j.ccr.2010.05.024. [27] SMITH B, NEFF R, COHN DE, et al. The mutational spectrum of FOXA2 in endometrioid endometrial cancer points to a tumor suppressor role[J]. Gynecol Oncol, 2016, 143(2): 398-405. DOI: 10.1016/j.ygyno.2016.08.237. [28] BASSERES DS, D'ALÒ F, YEAP BY, et al. Frequent downregulation of the transcription factor Foxa2 in lung cancer through epigenetic silencing[J]. Lung Cancer, 2012, 77(1): 31-37. DOI: 10.1016/j.lungcan.2012.01.011. [29] LI C, LU S, SHI Y. MicroRNA-187 promotes growth and metastasis of gastric cancer by inhibiting FOXA2[J]. Oncol Rep, 2017, 37(3): 1747-1755. DOI: 10.3892/or.2017.5370. [30] DING B, LIANG H, GAO M, et al. Forkhead Box A2 (FOXA2) inhibits invasion and tumorigenesis in glioma cells[J]. Oncol Res, 2017, 25(5): 701-708. DOI: 10.3727/096504016X14772378087005. [31] WANG J, ZHU CP, HU PF, et al. FOXA2 suppresses the metastasis of hepatocellular carcinoma partially through matrix metalloproteinase-9 inhibition[J]. Carcinogenesis, 2014, 35(11): 2576-2583. DOI: 10.1093/carcin/bgu180. [32] LIU J, YU Z, XIAO Y, et al. Coordination of FOXA2 and SIRT6 suppresses the hepatocellular carcinoma progression through ZEB2 inhibition[J]. Cancer Manag Res, 2018, 10: 391-402. DOI: 10.2147/CMAR.S150552. [33] PÉREZ-SÁNCHEZ C, ARIAS-DE-LA-FUENTE C, GÓMEZ-FERRERíA MA, et al. FHX. L and FHX. S, two isoforms of the human fork-head factor FHX (FOXJ2) with differential activity[J]. J Mol Biol, 2000, 301(4): 795-806. DOI: 10.1006/jmbi.2000.3999. [34] GRANADINO B, ARIAS-DE-LA-FUENTE C, PÉREZ-SÁNCHEZ C, et al. Fhx (Foxj2) expression is activated during spermatogenesis and very early in embryonic development[J]. Mech Dev, 2000, 97(1-2): 157-160. DOI: 10.1016/s0925-4773(00)00410-x. [35] GÓMEZ-FERRERÍA MA, REY-CAMPOS J. Functional domains of FOXJ2[J]. J Mol Biol, 2003, 329(4): 631-644. DOI: 10.1016/s0022-2836(03)00524-2. [36] MARTÍN-DE-LARA F, SÁNCHEZ-APARICIO P, ARIAS DE LA FUENTE C, et al. Biological effects of FoxJ2 over-expression[J]. Transgenic Res, 2008, 17(6): 1131-1141. DOI: 10.1007/s11248-008-9214-3. [37] QIU X, JI B, YANG L, et al. The role of FoxJ2 in the migration of human glioma cells[J]. Pathol Res Pract, 2015, 211(5): 389-397. DOI: 10.1016/j.prp.2015.01.005. [38] YANG Q, CAO X, TAO G, et al. Effects of FOXJ2 on TGF-β1-induced epithelial-mesenchymal transition through Notch signaling pathway in non-small lung cancer[J]. Cell Biol Int, 2017, 41(1): 79-83. DOI: 10.1002/cbin.10680. [39] WANG Y, YANG S, NI Q, et al. Overexpression of forkhead box J2 can decrease the migration of breast cancer cells[J]. J Cell Biochem, 2012, 113(8): 2729-2737. DOI: 10.1002/jcb.24146. [40] ZHANG Z, MENG G, WANG L, et al. The prognostic role and reduced expression of FOXJ2 in human hepatocellular carcinoma[J]. Mol Med Rep, 2016, 14(1): 254-262. DOI: 10.3892/mmr.2016.5261. -



 PDF下载 ( 3112 KB)
PDF下载 ( 3112 KB)

 下载:
下载: