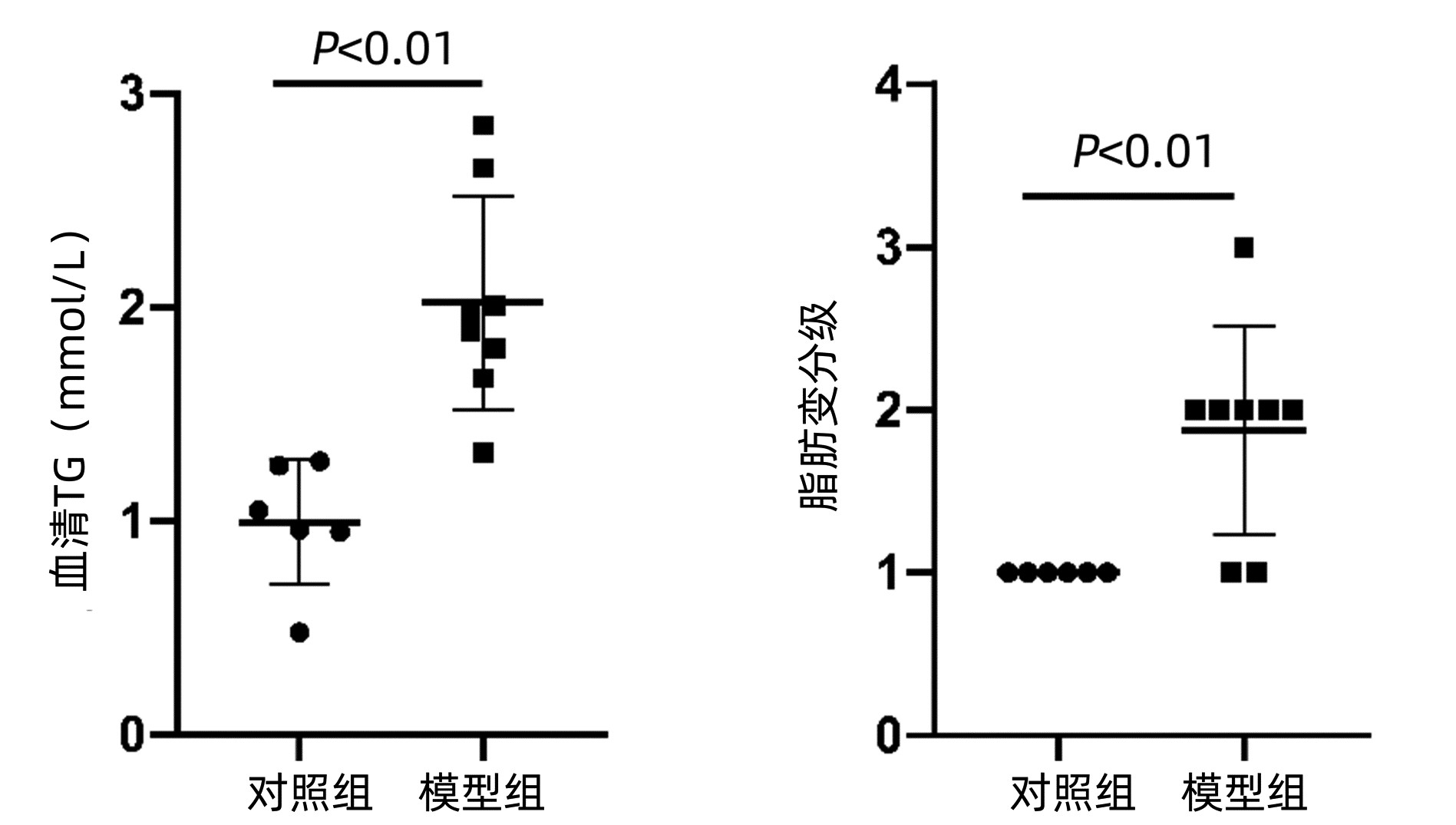
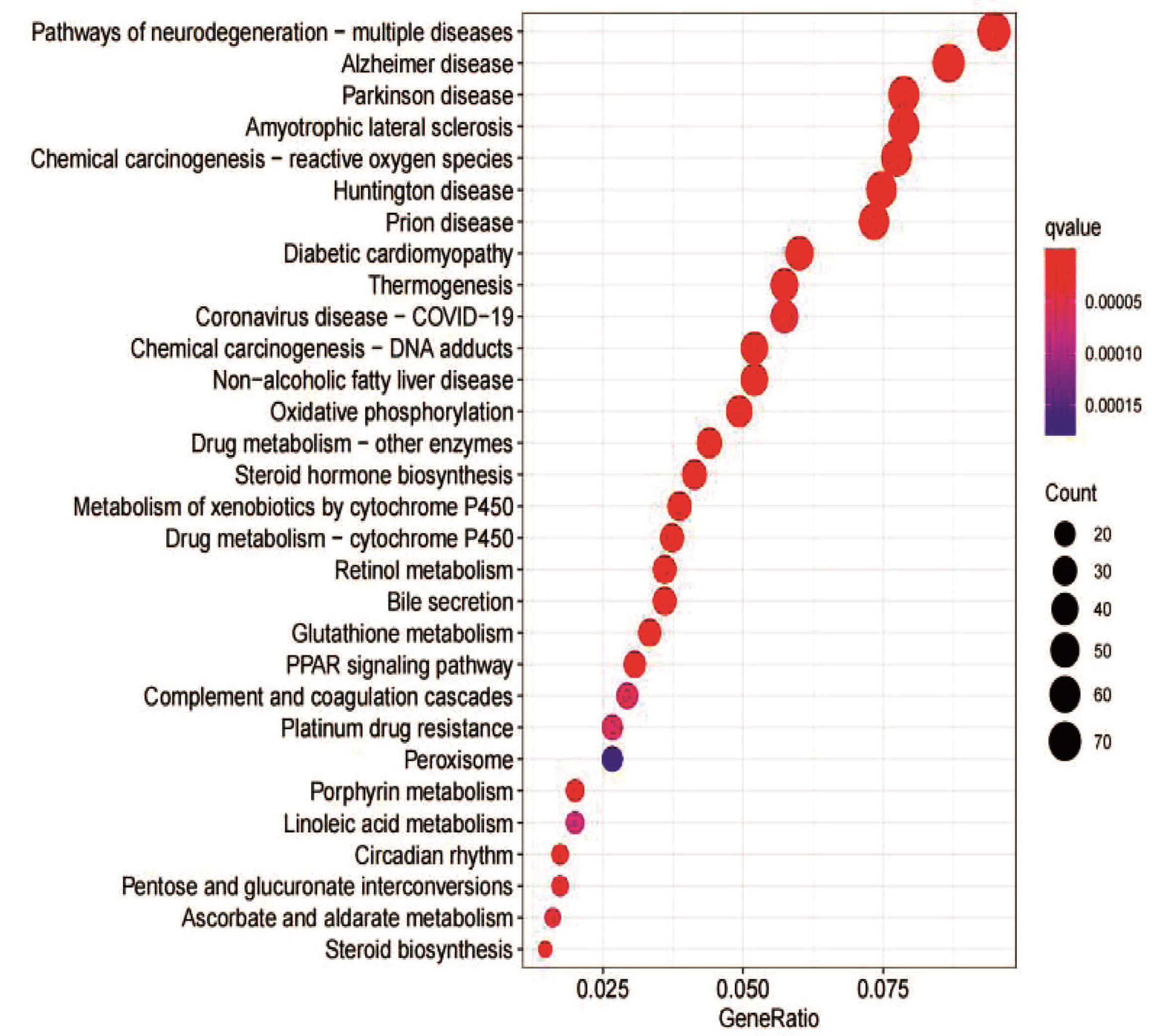
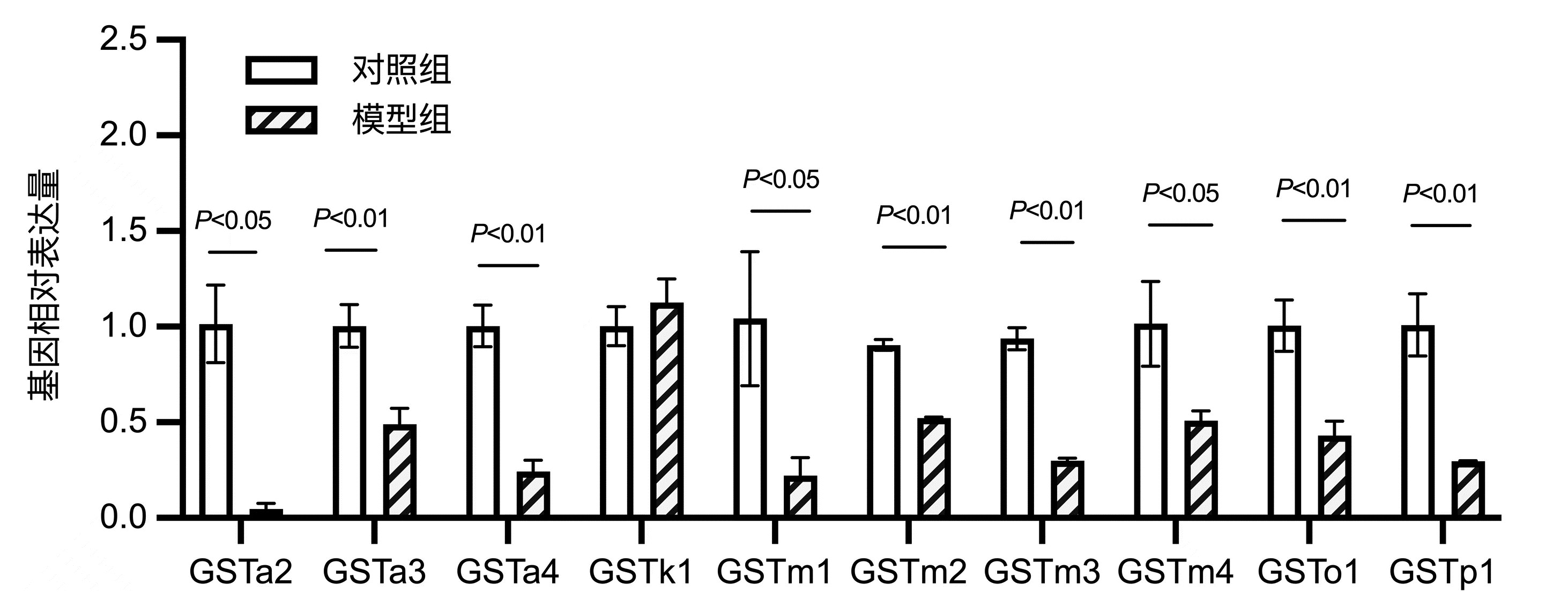
| [1] |
National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. |
| [2] |
LAN T, HU Y, HU F, et al. Hepatocyte glutathione S-transferase mu 2 prevents non-alcoholic steatohepatitis by suppressing ASK1 signaling[J]. J Hepatol, 2022, 76(2): 407-419. DOI: 10.1016/j.jhep.2021.09.040. |
| [3] |
HAYES PC, BOUCHIER IA, BECKETT GJ. Glutathione S-transferase in humans in health and disease[J]. Gut, 1991, 32(7): 813-818. DOI: 10.1136/gut.32.7.813. |
| [4] |
CAI QF, WU Q. Research progress of glutathione transferase[J]. J Hanan Med Coll, 2011, 17(12): 1735-1738. DOI: 46-1049/R.20111011.1408.007. |
| [5] |
MORRIS MJ, LIU D, WEAVER LM, et al. A structural basis for cellular uptake of GST-fold proteins[J]. PLoS One, 2011, 6(3): e17864. DOI: 10.1371/journal.pone.0017864. |
| [6] |
|
| [7] |
National Workshop on Fatty Liver and Alcoholic Liver Disease. Guidelines for diagnosis and treatment of nonalcoholic fatty liver disease (revised in February 2006)[J]. Mod Digest Interv, 2007, 12(4): 266-268. DOI: 10.3969/j.issn.1672-2159.2007.04.020. |
| [8] |
ZHU GR, WANG J, HUANG K, et al. A transcriptomic analysis of acute hepatotoxicity induced by aristolochic acidⅠin mice[J]. J Clin Hepatol, 2021, 37(10): 2389-2394. DOI: 10.3969/j.issn.1001-5256.2021.10.026. |
| [9] |
PRYSYAZHNYUK V, SYDORCHUK L, SYDORCHUK R, et al. Glutathione-S-transferases genes-promising predictors of hepatic dysfunction[J]. World J Hepatol, 2021, 13(6): 620-633. DOI: 10.4254/wjh.v13.i6.620. |
| [10] |
|
| [11] |
NEBERT DW, VASILIOU V. Analysis of the glutathione S-transferase (GST) gene family[J]. Hum Genomics, 2004, 1(6): 460-464. DOI: 10.1186/1479-7364-1-6-460. |
| [12] |
PRYSYAZHNYUK VP, ROSSOKHA ZI, GOROVENKO NG. Variation in particular biochemical indicators, cytokine and adipokine profiles of the blood, and the structural and functional parameters of the liver in patients with nonalcoholic fatty liver disease and different genotypes by the polymorphic locus A313G of the GSTP1 gene[J]. Cytol Genet, 2017, 6: 50-57. DOI: 10.3103/S0095452717060111. |
| [13] |
HASHEMI M, ESKANDARI-NASAB E, FAZAELI A, et al. Association of genetic polymorphisms of glutathione-S-transferase genes (GSTT1, GSTM1, and GSTP1) and susceptibility to nonalcoholic fatty liver disease in Zahedan, Southeast Iran[J]. DNA Cell Biol, 2012, 31(5): 672-677. DOI: 10.1089/dna.2011.1343. |
| [14] |
KASER S, MOSCHEN A, KASER A, et al. Circulating adiponectin reflects severity of liver disease but not insulin sensitivity in liver cirrhosis[J]. J Intern Med, 2005, 258(3): 274-280. DOI: 10.1111/j.1365-2796.2005.01543.x. |
| [15] |
PRYSYAZHNYUK V, VOLOSHYN O, PRYSIAZHNIUK I, et al. Glutathione S-transferase T1 and M1 null genotype distribution among non-alcoholic fatty liver disease patients and its association with cytokine and adipokine profiles[J]. Clin Exp Hepatol, 2020, 6(2): 142-149. DOI: 10.5114/ceh.2020.95678. |
| [16] |
RONIS M, MERCER K, ENGI B, et al. Global deletion of glutathione S-transferase A4 exacerbates developmental nonalcoholic steatohepatitis[J]. Am J Pathol, 2017, 187(2): 418-430. DOI: 10.1016/j.ajpath.2016.10.022. |
| [17] |
LI N, SUN YR, HE LB, et al. Amelioration by idesia polycarpa maxim. var. vestita diels. of oleic acid-induced nonalcoholic fatty liver in HepG2 cells through antioxidant and modulation of lipid metabolism[J]. Oxid Med Cell Longev, 2020, 2020: 1208726. DOI: 10.1155/2020/1208726. |
| [18] |
ZHU YL, ZHANG SJ, YANG C, et al. Quantitative analysis of differential proteins in liver tissues of patients with non-alcoholic steatohepatitis using iTRAQ technology[J]. J South Med Univ, 2021, 41(9): 1381-1387. DOI: 10.12122/j.issn.1673-4254.2021.09.13. |








 DownLoad:
DownLoad: