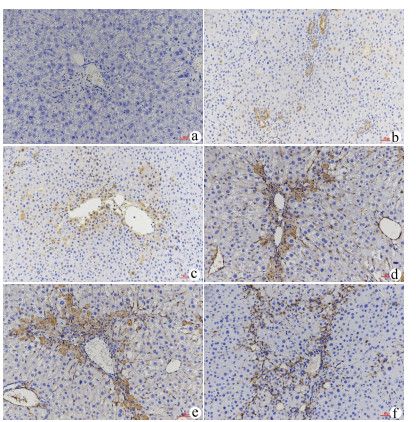
| [1] |
DU QH, ZHANG CJ, LI WH, et al. Gan Shen Fu Fang ameliorates liver fibrosis in vitro and in vivo by inhibiting the inflammatory response and extracellular signal-regulated kinase phosphorylation[J]. World J Gastroenterol, 2020, 26(21): 2810-2820. DOI: 10.3748/wjg.v26.i21.2810. |
| [2] |
TRAUTWEIN C, FRIEDMAN SL, SCHUPPAN D, et al. Hepatic fibrosis: Concept to treatment[J]. J Hepatol, 2015, 62(1 Suppl): s15-s24. DOI: 10.1016/j.jhep.2015.02.039. |
| [3] |
WU X, DONG L, LIN X, et al. Relevance of the NLRP3 inflammasome in the pathogenesis of chronic liver disease[J]. Front Immunol, 2017, 8: 1728. DOI: 10.3389/fimmu.2017.01728. |
| [4] |
HE K, ZHU X, LIU Y, et al. Inhibition of NLRP3 inflammasome by thioredoxin-interacting protein in mouse Kupffer cells as a regulatory mechanism for non-alcoholic fatty liver disease development[J]. Oncotarget, 2017, 8(23): 37657-37672. DOI: 10.18632/oncotarget.17489. |
| [5] |
CUI K, YAN G, XU C, et al. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1β in mice[J]. J Hepatol, 2015, 62(6): 1311-1318. DOI: 10.1016/j.jhep.2014.12.027. |
| [6] |
WU X, ZHANG F, XIONG X, et al. Tetramethylpyrazine reduces inflammation in liver fibrosis and inhibits inflammatory cytokine expression in hepatic stellate cells by modulating NLRP3 inflammasome pathway[J]. IUBMB Life, 2015, 67(4): 312-321. DOI: 10.1002/iub.1348. |
| [7] |
LENTSCH AB, KATO A, YOSHIDOME H, et al. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury[J]. Hepatology, 2000, 32(2): 169-173. DOI: 10.1053/jhep.2000.9323. |
| [8] |
SHARAWY MH, ABDEL-RAHMAN N, MEGAHED N, et al. Paclitaxel alleviates liver fibrosis induced by bile duct ligation in rats: Role of TGFβ1, IL-10 and c-Myc[J]. Life Sci, 2018, 211: 245-251. DOI: 10.1016/j.lfs.2018.09.037. |
| [9] |
Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and therapy of hepatic fibrosis(2019)[J]. J Clin Hepatol, 2019, 35(10): 2163-2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007. |
| [10] |
TAG CG, SAUER-LEHNEN S, WEISKIRCHEN S, et al. Bile duct ligation in mice: Induction of inflammatory liver injury and fibrosis by obstructive cholestasis[J]. J Vis Exp, 2015, 96: 52438. DOI: 10.3791/52438. |
| [11] |
ELPEK GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update[J]. World J Gastroenterol, 2014, 20(23): 7260-7276. DOI: 10.3748/wjg.v20.i23.7260. |
| [12] |
ZHOU WC, ZHANG QB, QIAO L. Pathogenesis of liver cirrhosis[J]. World J Gastroenterol, 2014, 20(23): 7312-7324. DOI: 10.3748/wjg.v20.i23.7312. |
| [13] |
CHANG J, LAN T, LI C, et al. Activation of Slit2-Robo1 signaling promotes liver fibrosis[J]. J Hepatol, 2015, 63(6): 1413-1420. DOI: 10.1016/j.jhep.2015.07.033. |
| [14] |
YANG F, LI LH. Effect of vitamin D receptor activation on hepatic fibrosis induced by bile duct ligation in mice and its mechanism[J]. J Jilin Univ(Med Edit), 2020, 46(4): 722-727. DOI: 10.13481/j.1671-587x.20200409. |
| [15] |
WU N, MENG F, ZHOU T, et al. The secretin/secretin receptor axis modulates ductular reaction and liver fibrosis through changes in transforming growth factor-β1-mediated biliary senescence[J]. Am J Pathol, 2018, 188(10): 2264-2280. DOI: 10.1016/j.ajpath.2018.06.015. |
| [16] |
GRESSNER AM, WEISKIRCHEN R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets[J]. J Cell Mol Med, 2006, 10(1): 76-99. DOI: 10.1111/j.1582-4934.2006.tb00292.x. |
| [17] |
CAO Z, WANG Y, LONG Z, et al. Interaction between autophagy and the NLRP3 inflammasome[J]. Acta Biochim Biophys Sin (Shanghai), 2019, 51(11): 1087-1095. DOI: 10.1093/abbs/gmz098. |
| [18] |
GROSLAMBERT M, PY BF. Spotlight on the NLRP3 inflammasome pathway[J]. J Inflamm Res, 2018, 11: 359-374. DOI: 10.2147/JIR.S141220. |
| [19] |
LAMKANFI M, DIXIT VM. Mechanisms and functions of inflammasomes[J]. Cell, 2014, 157(5): 1013-1022. DOI: 10.1016/j.cell.2014.04.007. |
| [20] |
STROWIG T, HENAO-MEJIA J, ELINAV E, et al. Inflammasomes in health and disease[J]. Nature, 2012, 481(7381): 278-286. DOI: 10.1038/nature10759. |
| [21] |
INZAUGARAT ME, JOHNSON CD, HOLTMANN TM, et al. NLR family pyrin domain-containing 3 inflammasome activation in hepatic stellate cells induces liver fibrosis in mice[J]. Hepatology, 2019, 69(2): 845-859. DOI: 10.1002/hep.30252. |
| [22] |
WANG H, LV C, WANG S, et al. NLRP3 Inflammasome involves in the acute exacerbation of patients with chronic obstructive pulmonary disease[J]. Inflammation, 2018, 41(4): 1321-1333. DOI: 10.1007/s10753-018-0780-0. |
| [23] |
WADA J, MAKINO H. Innate immunity in diabetes and diabetic nephropathy[J]. Nat Rev Nephrol, 2016, 12(1): 13-26. DOI: 10.1038/nrneph.2015.175. |
| [24] |
LIU D, ZENG X, LI X, et al. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases[J]. Basic Res Cardiol, 2018, 113(1): 5. DOI: 10.1007/s00395-017-0663-9. |
| [25] |
ZHEN Y, ZHANG H. NLRP3 Inflammasome and inflammatory bowel disease[J]. Front Immunol, 2019, 10: 276. DOI: 10.3389/fimmu.2019.00276. |
| [26] |
ZHANG N, YAN N, ZHOU ME, et al. Development and treatment of NLRP3 inflammatory corpuscule in acute and chronic liver diseases[J]. Liaoning J Trad Chin Med, 2020, 47(5): 217-220. DOI: 10.13192/j.issn.1000-1719.2020.05.065. |
| [27] |
MRIDHA AR, WREE A, ROBERTSON A, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice[J]. J Hepatol, 2017, 66(5): 1037-1046. DOI: 10.1016/j.jhep.2017.01.022. |
| [28] |
GONG Z, ZHOU J, ZHAO S, et al. Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis[J]. Oncotarget, 2016, 7(51): 83951-83963. DOI: 10.18632/oncotarget.13796. |








 DownLoad:
DownLoad: