三维可视化消融辅助系统在肝细胞癌射频消融术中的应用价值
DOI: 10.3969/j.issn.1001-5256.2022.09.019
Application of the three-dimensional visualization ablation planning system in radiofrequency ablation for hepatocellular carcinoma
-
摘要:
目的 探讨三维可视化消融辅助系统在肝细胞癌射频消融术中应用的意义。 方法 选取2017年7月—2020年12月于首都医科大学附属北京地坛医院因肝细胞癌行射频消融治疗的患者71例,其中三维组(n=34)术前应用三维可视化消融辅助系统进行射频方案规划,二维组(n=37)术前应用二维影像进行射频方案规划。比较两组患者的一次穿刺成功率、肿瘤完全消融率、无瘤生存期等。计数资料两组间比较选择Fisher精确检验、连续校正的χ2检验或Pearson χ2检验;符合正态分布的计量资料两组间比较采用t检验,非正态分布的计量资料两组间比较采用Mann-Whitney U检验;Kaplan-Meier法绘制生存曲线,Log-rank(Mantel-Cox)检验比较肿瘤复发和生存情况,通过Cox比例风险回归模型分析无瘤生存期的影响因素。 结果 三维组一次性穿刺成功率(94.12%)高于二维组(75.68%)(Pearson χ2=4.183,P=0.041),三维组术中穿刺耗时中位时间(5 min)低于二维组(7 min)(Z=-2.407,P=0.013)。三维组和二维组的消融完全率分别为97.06%和91.89%,两组差异无统计学意义(连续矫正χ2=0.183,P=0.669)。在1、2、4年累积无瘤生存率比较中,三维组分别为90.8%、78.8%和72.8%,二维组分别为61.5%、55.9%和44.7%, 两组差异有统计学意义(χ2=5.073,P=0.024)。多因素Cox回归分析结果显示,术前规划方式、消融是否完全、术后1个月AFP水平为肝细胞癌患者射频消融术后无瘤生存期的独立影响因素(P值均<0.05)。 结论 通过三维可视化消融辅助系统进行肝细胞癌射频消融规划,可以保证射频消融的疗效,降低肝细胞癌复发率,延长患者的无瘤生存期。 Abstract:Objective To investigate the significance of the three-dimensional visualization ablation planning system in radiofrequency ablation for liver cancer. Methods A total of 71 patients who received radiofrequency ablation for hepatocellular carcinoma in Beijing Ditan Hospital, Capital Medical University from July 2017 to December 2020 were enrolled as subjects. The 34 patients in the three-dimensional group used the three-dimensional visualization ablation planning system for radiofrequency protocol planning before surgery and the 37 patients in the two-dimensional group used the two-dimensional image for radiofrequency protocol planning before surgery. The two groups were compared in terms of the indices such as the first-attempt success rate of puncture, complete tumor ablation rate, and tumor-free survival. The Fisher's exact test, the chi-square test of continuous correction, or the Pearson chi-square test was used for comparison of categorical data between two groups; the t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups. The Kaplan-Meier method was used to plot survival curves, and the Log-rank (Mantel-Cox) test was used for comparison of tumor recurrence and survival; the Cox proportional-hazards regression model analysis was used to investigate the influencing factors for tumor-free survival. Results Compared with the two-dimensional group, the three-dimensional group had a significantly higher first-attempt success rate of puncture (94.12% vs 75.68%, Pearson χ2=4.183, P=0.041) and a significantly shorter median time of puncture (5 minutes vs 7 minutes, Z=-2.407, P=0.013). There was no significant difference in complete ablation rate between the three-dimensional group and the two-dimensional group (97.06% vs 91.89%, continuous correction χ2=0.183, P=0.669). There were significant differences in the 1-, 2-, and 4-year cumulative tumor-free survival rates between the three-dimensional group and the two-dimensional group (90.8%/78.8%/72.8% vs 61.5%/55.9%/44.7%, χ2=5.073, P=0.024). The multivariate Cox regression analysis showed that preoperative planning method, complete or incomplete ablation, and alpha-fetoprotein at 1 month after surgery were independent influencing factors for the tumor-free survival of patients with liver cancer after radiofrequency ablation (all P < 0.05). Conclusion Radiofrequency ablation planning via the three-dimensional visualization ablation planning system can ensure the therapeutic effect of radiofrequency ablation, reduce the recurrence rate of liver cancer, and prolong the tumor-free survival of patients. -
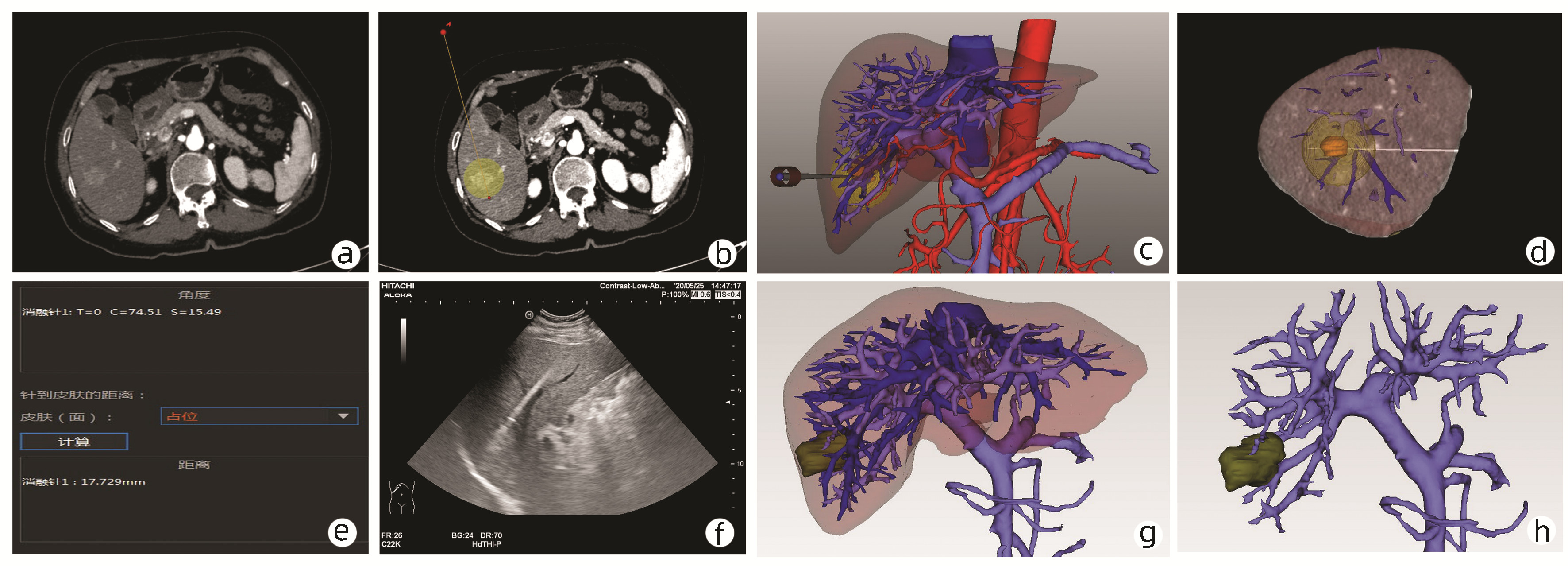
图 2 三维可视化消融辅助系统对肝S6段肿瘤RFA术前规划及术后效果评价
注:a, 二维影像显示肿瘤;b, 二维影像下选择射频路径和范围; c, 三维重建下消融球与穿刺路径; d, 模拟超声影像;e, 射频针与矢状面、冠状面、水平面的角度信息;f, RFA术中超声影像;g、h, 术后1个月复查实际消融范围。
Figure 2. Preoperative planning and postoperative outcomes of the radiofrequency ablation of the tumor in the S6 of the liver use the three-dimensional visualization ablation planning system
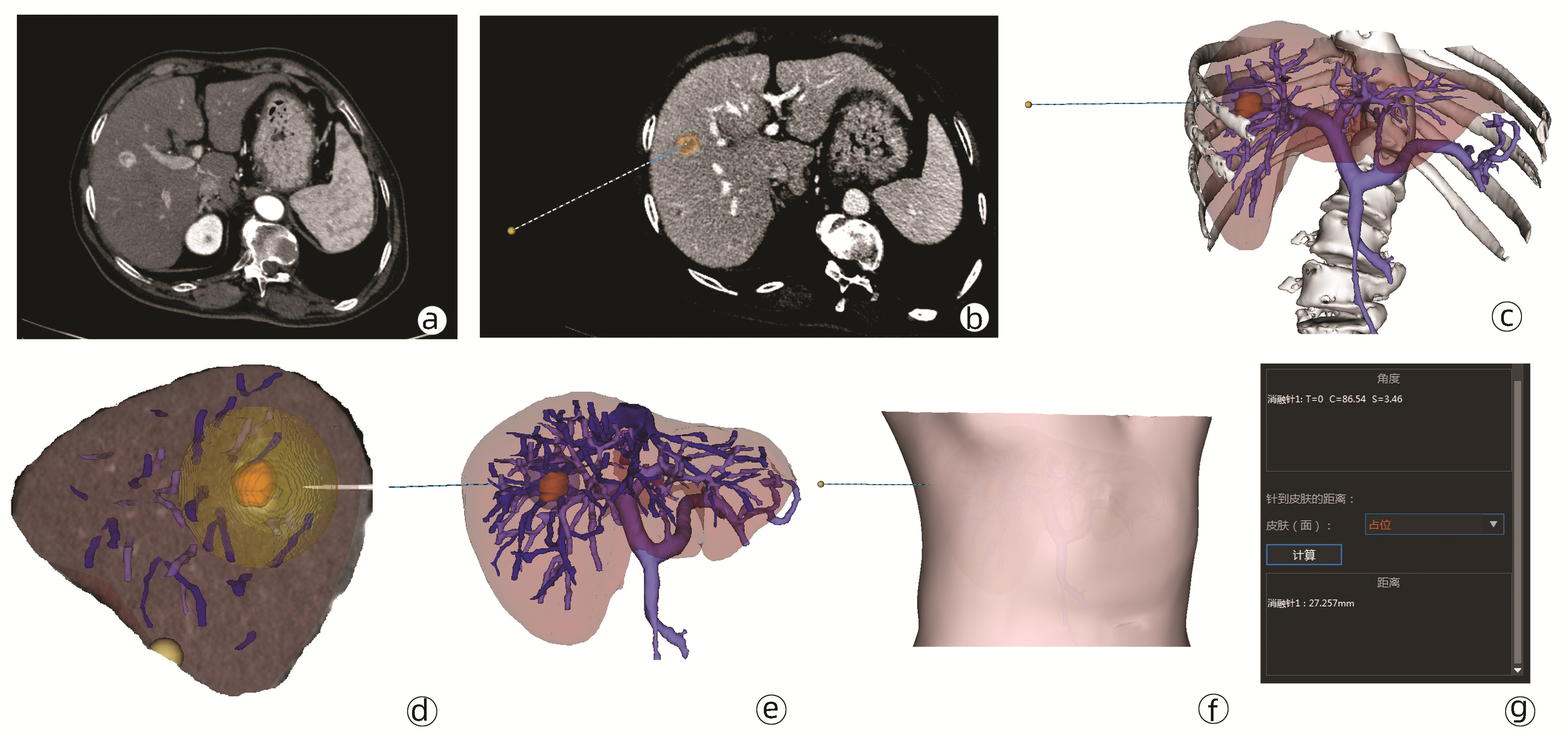
图 3 三维可视化消融辅助系统在肝S7段两枚肿瘤RFA的术前规划和术后评价
注:a、b, 肝S7段两枚肿瘤;c、d, 二维影像下选择消融范围及穿刺路径; e、f, 三维重建下显示消融范围、穿刺路径及消融球与周围血管关系;g、h, 术后1个月复查显示实际消融范围。
Figure 3. Preoperative planning and postoperative outcomes of the radiofrequency ablation of two tumors in the S7 of the liver use the three-dimensional visualization ablation planning system
表 1 两组间一般资料比较
Table 1. Baseline characteristics between the two groups
项目 三维组(n=34) 二维组(n=37) 统计值 P值 性别[例(%)] χ2=0.265 0.607 男 24(70.59) 24(64.86) 女 10(29.41) 13(35.14) 年龄(岁) 58.53±9.22 58.43±8.22 t=0.047 0.963 乙型肝炎(是/否,例) 28/6 28/9 χ2=0.474 0.491 肝硬化(是/否,例) 26/8 31/6 χ2=0.599 0.439 Child-Pugh评分 5(5~6) 5(5~6) Z=-0.144 0.885 MELD评分 8(7~9) 8(7~10) Z=-0.557 0.578 术前白细胞计数(×109/L) 4.74±1.81 4.47±1.53 t=0.678 0.500 术前血小板计数(×109/L) 111.50(70.93~138.25) 113.00(67.90~163.50) Z=-0.242 0.809 术前ALT(U/L) 25.20(14.65~35.77) 22.00(17.05~31.50) Z=-0.121 0.904 术前TBil(μmol/L) 15.15(11.98~18.73) 13.10(10.70~18.55) Z=-0.771 0.441 术前Alb(mg/L) 162.39±55.74 162.29±61.90 t=0.007 0.995 术前INR 1.12(1.04~1.19) 1.13(1.07~1.25) Z=-0.720 0.472 术前AFP(ng/mL) 10.35(3.05~59.65) 6.50(2.47~40.83) Z=-0.852 0.394 肿瘤数量(1/2/3,个) 28/5/1 34/1/2 0.172 肿瘤最大径(cm) 1.60(1.40~2.50) 1.90(1.50~2.40) Z=-1.085 0.278 手术方式[例(%)] χ2=3.408 0.065 经皮射频 22(64.7) 31(83.8) 经腹腔镜射频 12(35.3) 6(16.2) 术后白细胞计数(×109/L) 7.62±2.92 6.81±2.95 t=1.168 0.247 术后血小板计数(×109/L) 96.85(69.25~127.28) 98.40(73.50~150.70) Z=-3.222 0.747 术后ALT(U/L) 106.45(59.23~169.95) 78.00(49.20~137.60) Z=-1.209 0.227 术后TBil(μmol/L) 19.10(15.13~25.83) 18.80(15.85~25.20) Z=-0.150 0.881 术后前白蛋白(mg/L) 127.02±54.00 139.42±58.82 t=-0.923 0.359 术后INR 1.21(1.14~1.29) 1.19(1.10~1.32) Z=-0.466 0.641 术后并发症分级(无/Ⅰ/Ⅱ/ⅢA/ ⅢB/ⅣA/ⅣB, 例) 30/2/2/0/0/0/0 29/5/2/1/0/0/0 0.588 术后1个月AFP(ng/mL) 3.90(2.65~9.85) 4.08(2.14~8.39) Z=-0.357 0.721 表 2 影响无瘤生存期的单因素Cox比例风险回归分析
Table 2. Univariable Cox proportional hazard models of the tumor free survival
项目 SE Wald值 自由度 P值 B值 术前规划方式 0.573 3.997 1 0.046 0.318 是否合并乙型肝炎 0.317 4.697 1 0.030 1.987 肿瘤最大径 0.388 4.761 1 0.029 0.429 消融完全 0.574 10.092 1 0.001 0.161 术后1个月AFP 0.003 9.880 1 0.002 1.010 表 3 影响无瘤生存期的多因素Cox比例风险回归分析
Table 3. Multivariable Cox proportional hazard models of the tumor free survival
项目 SE Wald值 自由度 P值 B值 95%CI 术前规划方式 0.504 3.905 1 0.048 0.369 0.138~0.992 消融完全 0.651 11.462 1 0.001 0.110 0.031~0.395 术后1个月AFP 0.002 9.635 1 0.002 1.006 1.002~1.009 -
[1] FERLAY J, SOERJOMATARAM I, DIKSHIT R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012[J]. Int J Cancer, 2015, 136(5): E359- E386. DOI: 10.1002/ijc.29210. [2] YUAN SX, ZHOU WP. Progress and hot spots of comprehensive treatment for primary liver cancer[J]. Chin J Dig Surg, 2021, 20(2): 163-170. DOI: 10.3760/cma.j.cn115610-20201211-00776.袁声贤, 周伟平. 原发性肝癌综合治疗的进展和热点[J]. 中华消化外科杂志, 2021, 20(2): 163-170. DOI: 10.3760/cma.j.cn115610-20201211-00776. [3] ZHOU M, WANG H, ZENG X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2019, 394(10204): 1145-1158. DOI: 10.1016/S0140-6736(19)30427-1. [4] Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [5] European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. [6] ZHU F, RHIM H. Thermal ablation for hepatocellular carcinoma: what's new in 2019[J]. Chin Clin Oncol, 2019, 8(6): 58. DOI: 10.21037/cco.2019.11.03. [7] LI R, CHEN HP, WANG F, et al. Application of contrast-enhanced ultrasound combined with percutaneous radiofrequency ablation in treatment of liver cancer rupture and bleeding[J]. Chin J Gerontol, 2020, 40(17): 3653-3656. DOI: 10.3969/j.issn.1005-9202.2020.17.021.李锐, 陈卉品, 王菲, 等. 超声造影联合经皮射频消融在肝癌破裂出血治疗中的应用[J]. 中国老年学杂志, 2020, 40(17): 3653-3656. DOI: 10.3969/j.issn.1005-9202.2020.17.021. [8] ZHI-YU H, PING L, XIAO-LING Y, et al. A clinical study of thermal monitoring techniques of ultrasound-guided microwave ablation for hepatocellular carcinoma in high-risk locations[J]. Sci Rep, 2017, 7: 41246. DOI: 10.1038/srep41246. [9] HUANG S, YU J, LIANG P, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up[J]. Eur J Radiol, 2014, 83(3): 552-558. DOI: 10.1016/j.ejrad.2013.12.015. [10] CAO F, FAN WJ. Ablation therapy for large hepatocellular carcinoma[J]. J Clin Hepatol, 2021, 37(3): 501-505. DOI: 10.3969/j.issn.1001-5256.2021.03.002.曹飞, 范卫君. 大肝癌的消融治疗[J]. 临床肝胆病杂志, 2021, 37(3): 501-505. DOI: 10.3969/j.issn.1001-5256.2021.03.002. [11] HU H, CHEN GF, YUAN W, et al. Microwave ablation with chemoembolization for large hepatocellular carcinoma in patients with cirrhosis[J]. Int J Hyperthermia, 2018, 34(8): 1351-1358. DOI: 10.1080/02656736.2018.1462536. [12] FANG CH, ZHANG P, ZHOU WP, et al. Efficacy of three-dimensional visualization technology in the precision diagnosis and treatment for primary liver cancer: a retrospective multicenter study of 1 665 cases in China[J]. Chin J Surg, 2020, 58(5): 375-382. DOI: 10.3760/cma.j.cn112139-20200220-00105.方驰华, 张鹏, 周伟平, 等. 三维可视化技术用于1665例原发性肝癌精准诊治的多中心回顾性研究[J]. 中华外科杂志, 2020, 58(5): 375-382. DOI: 10.3760/cma.j.cn112139-20200220-00105. [13] BARI H, WADHWANI S, DASARI B. Role of artificial intelligence in hepatobiliary and pancreatic surgery[J]. World J Gastrointest Surg, 2021, 13(1): 7-18. DOI: 10.4240/wjgs.v13.i1.7. [14] YEO CT, MACDONALD A, UNGI T, et al. Utility of 3D reconstruction of 2D liver computed tomography/magnetic resonance images as a surgical planning tool for residents in liver resection surgery[J]. J Surg Educ, 2018, 75(3): 792-797. DOI: 10.1016/j.jsurg.2017.07.031. [15] LI SS, ZHANG K, CHENG SJ, et al. Current status and future perspectives of the application of medical 3D visualization technology in accurate surgery of liver tumors[J]. J Clin Hepatol, 2019, 35(5): 1114-1117. DOI: 10.3969/j.issn.1001-5256.2019.05.042.李山山, 张珂, 程树杰, 等. 医学三维可视化技术在肝肿瘤精准手术中的应用现状及展望[J]. 临床肝胆病杂志, 2019, 35(5): 1114-1117. DOI: 10.3969/j.issn.1001-5256.2019.05.042. [16] HU M, HU H, CAI W, et al. The safety and feasibility of three-dimensional visualization technology assisted right posterior lobe allied with part of V and VⅢ sectionectomy for right hepatic malignancy therapy[J]. J Laparoendosc Adv Surg Tech A, 2018, 28(5): 586-594. DOI: 10.1089/lap.2017.0479. [17] RENZULLI M, TOVOLI F, CLEMENTE A, et al. Ablation for hepatocellular carcinoma: beyond the standard indications[J]. Med Oncol, 2020, 37(4): 23. DOI: 10.1007/s12032-020-01348-y. [18] YOSHIDA S, KORNEK M, IKENAGA N, et al. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma[J]. Hepatology, 2013, 58(5): 1667-1680. DOI: 10.1002/hep.26526. [19] CROCETTI L, de BAÉRE T, PEREIRA PL, et al. CIRSE standards of practice on thermal ablation of liver tumours[J]. Cardiovasc Intervent Radiol, 2020, 43(7): 951-962. DOI: 10.1007/s00270-020-02471-z. [20] YANG JD, HAINAUT P, GORES GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management[J]. Nat Rev Gastroenterol Hepatol, 2019, 16(10): 589-604. DOI: 10.1038/s41575-019-0186-y. -



 PDF下载 ( 4804 KB)
PDF下载 ( 4804 KB)

 下载:
下载: