中晚期肝细胞癌靶向联合免疫治疗进展
DOI: 10.3969/j.issn.1001-5256.2022.05.004
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:刘秀峰对研究的思路有关键贡献,参与了研究数据的获取分析,并起草和修改文章关键内容;张珏、姚琳参与起草和修改文章关键内容;杨朝旭对研究的思路和设计有关键贡献。
Advances in targeted therapy combined with immunotherapy for advanced hepatocellular carcinoma
-
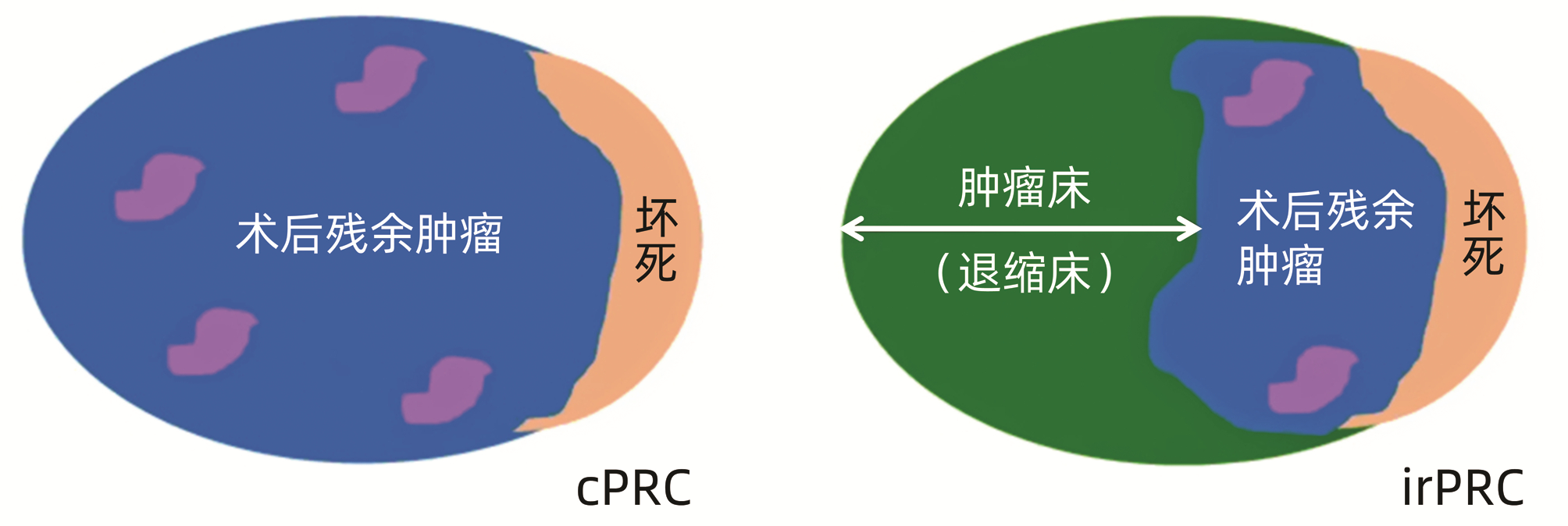
摘要: IMbrave 150研究开启了免疫治疗联合靶向治疗的大门,随之针对中国患者的Ⅲ期临床研究ORIENTAL-32结果公布,印证了免疫联合靶向的疗效,尤其针对中国患者,生存获益显著。目前诸多程序性死亡受体1/程序性死亡受体-配体1抑制剂联合小分子酪氨酸激酶抑制剂的研究正在进行中,其相应的早期数据提供了非常可观的客观缓解率,为不同病期、不同阶段肝细胞癌的转化治疗/序贯治疗提供了契机,也为新辅助/辅助治疗的深入探索提供了基础。免疫联合治疗已进入3.0版时代,在联合靶向药物的同时,可以在不同阶段实施合理的局部治疗,但目前尚无精准的疗效预测指标,需要缜密结合疾病的自然病程、临床病理学参数、基因组学、影像组学等特征综合考量。免疫联合治疗较之单药免疫或单药靶向治疗,不良反应谱相对复杂,相关性鉴别困难,需要多学科综合诊疗框架下的全程管理、综合判断和及时处置。Abstract: The IMbrave 150 study opened the door of immunotherapy combined with targeted therapy, and then the data of ORIENTAL-32, a Phase Ⅲ clinical trial for Chinese patients, was released, which confirmed the efficacy of immunotherapy combined with targeted therapy, especially significant survival benefits in Chinese patients. At present, there are many ongoing studies on PD-1/PD-L1 inhibitors combined with small-molecule tyrosine kinase inhibitors, and their corresponding early data provide a considerable objective response rate, which provides an opportunity for conversion therapy/sequential therapy for hepatocellular carcinoma in different stages and courses, as well as a basis for further exploration of neoadjuvant/adjuvant therapy. Combined immunotherapy has entered the era of version 3.0, in which reasonable local therapy can be implemented at different stages in combination with targeted drugs. However, there are still no accurate predictive indicators for efficacy, and it requires comprehensive consideration of the features such as the natural course of the disease, clinicopathological parameters, genomics, and radiomics. Compared with single-drug immunotherapy or single-drug targeted therapy, immunotherapy combined with targeted therapy had a relatively complex spectrum of adverse reactions and difficult identification of correlation, and whole-process management, comprehensive judgment, and timely treatment should be performed within the framework of multidisciplinary team.
-
Key words:
- Carcinoma, Hepatocellular /
- Molecular Targeted Therapy /
- Immunotherapy
-
-
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. [2] ZHOU M, WANG H, ZENG X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2019, 394(10204): 1145-1158. DOI: 10.1016/S0140-6736(19)30427-1. [3] National Health Commission of the People's Republic of China Medical Administration and Hospital Administration. Guidelines for the diagnosis and treatment of primary liver cancer (2022 edition)[OL]. http://www.nhc.gov.cn/yzygj/s7659/202201/a01ceb75c62b486fa459e36ba0fdfdbc.shtml.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗指南(2022年版)[OL]. http://www.nhc.gov.cn/yzygj/s7659/202201/a01ceb75c62b486fa459e36ba0fdfdbc.shtml. [4] NCCN Clinical Practice Guidelines in Oncology(NCCN Guidelines®). Hepatobiliary Cancers. Version 5.2021[OL]. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. [5] Chinese Society of Clinical Oncology Guidelines Working Committee. Chinese Society of Clinical Oncology(CSCO) guidelines for the diagnosis and treatment of primary liver cancer 2020[M]. Beijing: People's Health Publishing House, 2020.中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)原发性肝癌诊疗指南2020[M]. 北京: 人民卫生出版社, 2020. [6] The Chinese Chapter of the International Hepato-Pancreato-Biliary Association; Group of Liver Surgery, Surgical Society of Chinese Medical Association; Expert Committee on Liver Cancer, Chinese Society of Clinical Oncology. Chinese multidisciplinary expert consensus on combined immunotherapy based on immune checkpoint inhibitors for hepatocellular carcinoma (2021 version) [J]. Chin J Dig Surg, 2021, 20(7): 740-753. DOI: 10.3760/cma.j.cn115610-20210603-00260.国际肝胆胰协会中国分会, 中华医学会外科学分会肝脏外科学组, 中国临床肿瘤学会(CSCO)肝癌专家委员会. 基于免疫节点抑制剂的肝细胞癌免疫联合治疗多学科中国专家共识(2021版)[J]. 中华消化外科杂志, 2021, 20(7): 740-753. DOI: 10.3760/cma.j.cn115610-20210603-00260. [7] LLOVET JM, KELLEY RK, VILLANUEVA A, et al. Hepatocellular carcinoma[J]. Nat Rev Dis Primers, 2021, 7(1): 6. DOI: 10.1038/s41572-020-00240-3. [8] HOSHIDA Y, VILLANUEVA A, KOBAYASHI M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma[J]. N Engl J Med, 2008, 359(19): 1995-2004. DOI: 10.1056/NEJMoa0804525. [9] CHENG AL, KANG YK, CHEN Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase Ⅲ randomised, double-blind, placebo-controlled trial[J]. Lancet Oncol, 2009, 10(1): 25-34. DOI: 10.1016/S1470-2045(08)70285-7. [10] KUDO M, FINN RS, QIN S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391(10126): 1163-1173. DOI: 10.1016/S0140-6736(18)30207-1. [11] QIN S, BI F, GU S, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: A randomized, open-label, parallel-controlled phase Ⅱ-Ⅲ trial[J]. J Clin Oncol, 2021, 39(27): 3002-3011. DOI: 10.1200/JCO.21.00163. [12] BRUIX J, QIN S, MERLE P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2017, 389(10064): 56-66. DOI: 10.1016/S0140-6736(16)32453-9. [13] QIN S, LI Q, GU S, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial[J]. Lancet Gastroenterol Hepatol, 2021, 6(7): 559-568. DOI: 10.1016/S2468-1253(21)00109-6. [14] ABOU-ALFA GK, MEYER T, CHENG AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma[J]. N Engl J Med, 2018, 379(1): 54-63. DOI: 10.1056/NEJMoa1717002. [15] FINN RS, QIN S, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20): 1894-1905. DOI: 10.1056/NEJMoa1915745. [16] ZHU AX, KANG YK, YEN CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Oncol, 2019, 20(2): 282-296. DOI: 10.1016/S1470-2045(18)30937-9. [17] EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389(10088): 2492-2502. DOI: 10.1016/S0140-6736(17)31046-2. [18] ZHU AX, FINN RS, EDELINE J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial[J]. Lancet Oncol, 2018, 19(7): 940-952. DOI: 10.1016/S1470-2045(18)30351-6. [19] QIN S, REN Z, MENG Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial[J]. Lancet Oncol, 2020, 21(4): 571-580. DOI: 10.1016/S1470-2045(20)30011-5. [20] REN Z, XU J, BAI Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study[J]. Lancet Oncol, 2021, 22(7): 977-990. DOI: 10.1016/S1470-2045(21)00252-7. [21] DUCREUX M, ABOU-ALFA GK, REN Z, et al. Results from a global Phase 2 study of tislelizumab, an investigational PD-1 antibody, in patients with previously treated advanced hepatocellular carcinoma[J]. Ann Oncol, 2021, 32(3s): s217. [22] YAU T, KANG YK, KIM TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial[J]. JAMA Oncol, 2020, 6(11): e204564. DOI: 10.1001/jamaoncol.2020.4564. [23] FINN RS, QIN S, IKEDA M, et al. IMbrave150: Updated overall survival data from a global, randomized, open-label Phase Ⅲ study of atezolizumab+bevacizumab vs sorafenib in patients with unresectable hepatocellular carcinoma[R]. J Clin Oncol, 2021, 39(3s): abstract 267. [24] FINN RS, IKEDA M, ZHU AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma[J]. J Clin Oncol, 2020, 38(26): 2960-2970. DOI: 10.1200/JCO.20.00808. [25] KELLEY RK, YAU T, CHENG AL, et al. Cabozantinib plus atezolizumab versus sorafenib as first-line systemic treatment for advanced hepatocellular carcinoma: Results from the randomized phase 3 COSMIC-312 trial[R]. Esmo Asia Virtual Plenary, 2021, Abstract VP10-2021. [26] LLOVET JM, VILLANUEVA A, MARRERO JA, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference[J]. Hepatology, 2021, 73(Suppl 1): 158-191. DOI: 10.1002/hep.31327. [27] LLOVET JM, LENCIONI R. mRECIST for HCC: Performance and novel refinements[J]. J Hepatol, 2020, 72(2): 288-306. DOI: 10.1016/j.jhep.2019.09.026. [28] SUN L, MU L, ZHOU J, et al. Imaging features of gadoxetic acid-enhanced MR imaging for evaluation of tumor-infiltrating CD8 cells and PD-L1 expression in hepatocellular carcinoma[J]. Cancer Immunol Immunother, 2022, 71(1): 25-38. DOI: 10.1007/s00262-021-02957-w. [29] KUDO M, UESHIMA K, NAKAHIRA S, et al. Adjuvant nivolumab for hepatocellular carcinoma (HCC) after surgical resection (SR) or radiofrequency ablation (RFA) (NIVOLVE): A phase 2 prospective multicenter single-arm trial and exploratory biomarker analysis[R]. 2021 ASCO, Abstract 4093. [30] LIAO H, ZHANG Z, CHEN J, et al. Preoperative radiomic approach to evaluate tumor-infiltrating CD8+ T cells in hepatocellular carcinoma patients using contrast-enhanced computed tomography[J]. Ann Surg Oncol, 2019, 26(13): 4537-4547. DOI: 10.1245/s10434-019-07815-9. [31] SUN HC, RAO SX, XU B, et al. Predicting the efficacy of lenvatinib plus anti-PD-1 antibodies in unresectable hepatocellular carcinoma (uHCC) using radiomics features of tumors extracted from baseline MRI[J]. Ann Oncol, 2021, 32(Suppl 5): 948P. [32] OGSTON KN, MILLER ID, PAYNE S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: Prognostic significance and survival[J]. Breast, 2003, 12(5): 320-327. DOI: 10.1016/s0960-9776(03)00106-1. [33] STEIN JE, LIPSON EJ, COTTRELL TR, et al. Pan-tumor pathologic scoring of response to PD-(L)1 blockade[J]. Clin Cancer Res, 2020, 26(3): 545-551. DOI: 10.1158/1078-0432.CCR-19-2379. [34] COTTRELL TR, THOMPSON ED, FORDE PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: A proposal for quantitative immune-related pathologic response criteria (irPRC)[J]. Ann Oncol, 2018, 29(8): 1853-1860. DOI: 10.1093/annonc/mdy218. [35] LEON-MATEOS L, GARCIA-VELLOSO MJ, GARCÍA-FIGUEIRAS R, et al. A multidisciplinary consensus on the morphological and functional responses to immunotherapy treatment[J]. Clin Transl Oncol, 2021, 23(3): 434-449. DOI: 10.1007/s12094-020-02442-3. [36] MARRON T, GUNASEKARAN G, TABRIZIAN P, et al. Neoadjuvant cemiplimab demonstrated complete pathological responses in hepatocelluler carcinoma[R]. 2021 AACR: CT182. [37] Alliance of Liver Cancer Conversion Therapy, Committee of Liver Cancer of the Chinese Anti-Cancer Association. Chinese expert consensus on conversion therapy in hepatocellular carcinoma (2021 edition)[J]. Chin J Dig Surg, 2021, 20(6): 600-616. DOI: 10.3760/cma.j.cn115610-20210512-00223.中国抗癌协会肝癌专业委员会转化治疗协作组. 肝癌转化治疗中国专家共识(2021版)[J]. 中华消化外科杂志, 2021, 20(6): 600-616. DOI: 10.3760/cma.j.cn115610-20210512-00223. [38] SONBOL MB, RIAZ IB, NAQVI S, et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: A systematic review and network meta-analysis[J]. JAMA Oncol, 2020, 6(12): e204930. DOI: 10.1001/jamaoncol.2020.4930. [39] GALLE PR, KIM RD, SUNG MW, et al. Updated results of a phase Ib study of regorafenib (REG) plus pembrolizumab (PEMBRO) for first-line treatment of advanced hepatocellular carcinoma (HCC)[R]. EAMO Virtual 2020: 990P [40] CHENG AL, HSU C, CHAN SL, et al. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma[J]. J Hepatol, 2020, 72(2): 307-319. DOI: 10.1016/j.jhep.2019.09.025. [41] STEIN S, HSU CH, LEE M, et al. Patterns of response to atezolizumab (atezo) + bevacizumab (bev) in hepatocellular carcinoma (HCC) from the Phase 1b GO30140 study[J]. Digital Liver Cancer Summit, 2021: 24. [42] BORCOMAN E, KANJANAPAN Y, CHAMPIAT S, et al. Novel patterns of response under immunotherapy[J]. Ann Oncol, 2019, 30(3): 385-396. DOI: 10.1093/annonc/mdz003. [43] SPAGNOLO F, BOUTROS A, CECCHI F, et al. Treatment beyond progression with anti-PD-1/PD-L1 based regimens in advanced solid tumors: A systematic review[J]. BMC Cancer, 2021, 21(1): 425. DOI: 10.1186/s12885-021-08165-0. [44] Chinese Society of Liver Cancer, Chinese Anti-Cancer Association. Chinese expert consensus on multidisciplinary treatment of liver cancer[J]. J Clin Hepatol, 2021, 37(2): 278-285. DOI: 10.3969/j.issn.1001-5256.2021.02.008.中国抗癌协会肝癌专业委员会. 中国肝癌多学科综合治疗专家共识[J]. 临床肝胆病杂志, 2021, 37(2): 278-285. DOI: 10.3969/j.issn.1001-5256.2021.02.008. -



 PDF下载 ( 3145 KB)
PDF下载 ( 3145 KB)


 下载:
下载: