CalliSpheres载药微球与传统经肝动脉化疗栓塞术治疗肝细胞癌效果和安全性比较的Meta分析
DOI: 10.3969/j.issn.1001-5256.2021.08.019
Efficacy and safety of CalliSpheres microsphere versus conventional transcatheter arterial chemoembolization in treatment of hepatocellular carcinoma: A Meta-analysis
-
摘要:
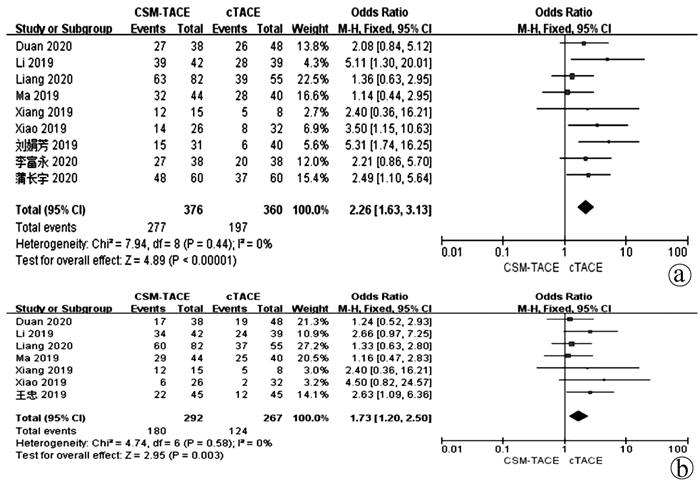
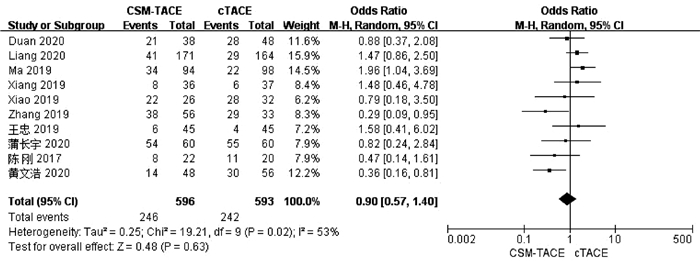
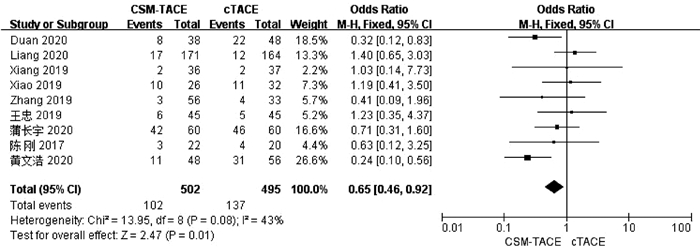
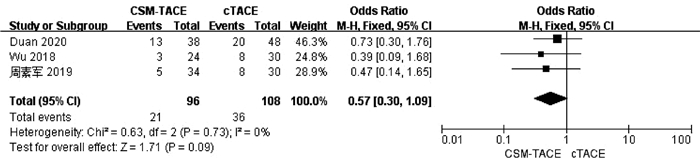
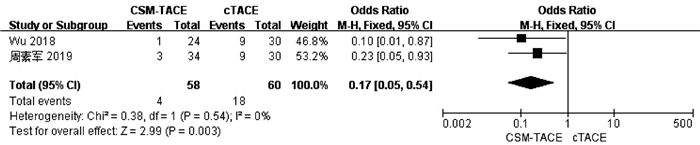
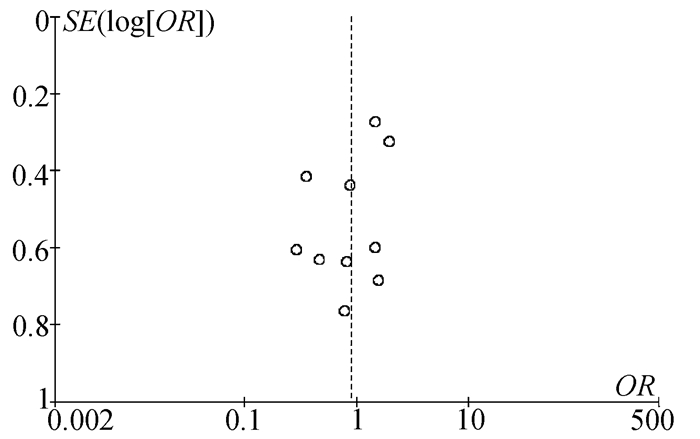
目的 本研究旨在通过Meta分析比较CalliSpheres载药微球经肝动脉化疗栓塞术(CSM-TACE)与传统经肝动脉化疗栓塞术(cTACE)治疗肝细胞癌的有效性和安全性。 方法 系统的检索PubMed、Web Science、Cochrane Library、中国知网数据库、万方数据库和维普数据库, 比较CSM-TACE与cTACE在肝细胞癌中应用的所有中英文文献, 截止日期到2020年10月。对纳入文献进行质量学评价后, 采用Cochrane Library提供的RevMan 5.3软件进行分析。 结果 经筛选后共纳入15篇研究, 包括1535例患者。Meta分析显示, 接受CSM-TACE治疗的患者1年总生存率(OR=2.26, 95%CI: 1.63~3.13, P<0.000 01)、2年总生存率(OR=1.73, 95%CI: 1.20~2.50, P=0.003) 和2年无进展生存率(OR=1.60, 95%CI: 1.05~2.43, P=0.03)显著高于接受cTACE治疗的患者。而且在安全性方面, 接受CSM-TACE治疗的患者术后呕吐率(OR=0.65, 95%CI: 0.46~0.92, P=0.01)、骨髓抑制率(OR=0.17, 95%CI: 0.05~0.54, P=0.003)、粒细胞减少率(OR=0.18, 95%CI: 0.07~0.45, P=0.000 3)均明显低于接受cTACE治疗的患者, 在术后发热率、腹痛率、腹水率方面两组差异均无统计学意义(P值均>0.05)。 结论 CSM-TACE在提高1年和2年总生存率以及2年无进展生存率方面具有显著优势, 而且可明显降低患者术后呕吐率、骨髓抑制率、粒细胞减少率。因此, CSM-TACE是一种安全、有效的治疗方式。 -
关键词:
- 癌, 肝细胞 /
- 微球体 /
- 化学栓塞, 治疗性 /
- Meta分析(主题)
Abstract:Objective To investigate the efficacy and safety of CalliSpheres microsphere-transcatheter arterial chemoembolization (CSM-TACE) versus conventional transcatheter arterial chemoembolization (cTACE) in the treatment of hepatocellular carcinoma (HCC) through a meta-analysis. Methods PubMed, Web of Science, Cochrane Library, CNKI, Wanfang Data, and VIP were searched for all Chinese and English articles on the application of CSM-TACE and cTACE in HCC published up to the end of October, 2020. After quality assessment was performed for the articles included, RevMan 5.3 software provided by Cochrane Library was used for analysis. Results A total of 15 studies were included, with 1535 patients in total. This meta-analysis showed that compared with the patients receiving cTACE, the patients receiving CSM-TACE had significantly higher 1-year overall survival rate (odds ratio [OR]=2.26, 95% confidence interval [CI]: 1.63-3.13, P < 0.000 01), 2-year overall survival rate (OR=1.73, 95%CI: 1.20-2.50, P=0.003), and 2-year progression-free survival rate (OR=1.60, 95%CI: 1.05-2.43, P=0.03). In terms of safety, compared with the patients receiving cTACE, the patients receiving CSM-TACE had significantly lower incidence rates of postoperative vomiting (OR=0.65, 95%CI: 0.46-0.92, P=0.01), bone marrow suppression (OR=0.17, 95%CI: 0.05-0.54, P=0.003), and neutropenia (OR=0.18, 95%CI: 0.07-0.45, P=0.000 3), while there were no significant differences between the two groups of patients in postoperative pyrexia, abdominal pain, and ascites (all P > 0.05). Conclusion CSM-TACE has significant advantages in improving 1- and 2-year overall survival rates and 2-year progression-free survival rates and can significantly reduce the incidence rates of postoperative vomiting, bone marrow suppression, and neutropenia. Therefore, CSM-TACE is a safe and effective treatment method. -
表 1 纳入文献的基本特征及质量评价
第一作者 治疗 样本量(例) 研究时间(年) 研究类型 性别(男/女, 例) BCLC分期(A/B/C+D, 例) 肿瘤大小(cm) PVTT (是/否, 例) Child-Pugh分级(A/B/C, 例) 结果 NOS评分 Wu 2018[9] CSM-TACE 24 2016—2017 回顾性 22/2 0/13/11 7.25±2.3 NA 10/14/0 ⑥⑦⑧ 7 cTACE 30 27/3 0/17/13 7.53±2.3 NA 16/14/0 黄文浩2020[10] CSM-TACE 48 2017—2019 回顾性 35/13 17/25/6 NA NA 28/20/0 ③④⑤ 8 cTACE 56 40/16 21/27/8 31/25/0 Ma 2019[13] CSM-TACE 94 NA 回顾性 78/16 22/32/40 9.6±1.4 35/59 69/24/1 ①②③④ 9 cTACE 98 87/11 23/40/35 7.5±1.2 29/69 75/22/1 Zhang 2019[14] CSM-TACE 56 2013— 2017 回顾性 45/11 0/8/48 11.2±6.6 47/9 36/20/0 ③④⑤ 8 cTACE 33 28/5 0/5/28 10.7±5.8 27/6 23/10/0 Xiao 2019[15] CSM-TACE 26 2010— 2016 回顾性 22/4 0/13/13 9.7±3.8 11/14 20/6/0 ①③④⑤ 8 cTACE 32 27/5 0/15/17 10.8±5.9 13/19 21/11/0 Xiang 2019[16] CSM-TACE 36 2015— 2017 回顾性 31/5 9/17/10 5.5±1.4 9/27 30/6/0 ①②③④⑤ 8 cTACE 37 33/4 13/18/6 5.6±1.5 5/32 30/7/0 Li 2019[17] CSM-TACE 42 2015— 2017 回顾性 37/5 5/22/15 7.8±3.2 11/31 36/6/0 ①② 7 cTACE 39 33/6 8/22/9 6.7±4.0 6/33 34/5/0 Duan 2020[18] CSM-TACE 38 2017— 2018 回顾性 37/1 0/20/18 9.4±4.3 20/18 25/13/0 ①②③④⑤⑥ 9 cTACE 48 46/2 0/25/23 9.9±4.2 27/21 35/13/0 Liang 2020[19] CSM-TACE 171 2014— 2017 回顾性 145/26 36/73/62 7.9±1.8 53/118 136/34/1 ①②③④⑤ 8 cTACE 164 146/18 40/73/51 6.5±1.1 38/126 133/29/2 周素军2019[20] CSM-TACE 34 2016—2017 回顾性 30/4 0/19/15 7.5±1.19 NA 18/16/0 ⑥⑦⑧ 8 cTACE 30 27/3 0/17/13 7.43±1.08 NA 16/14/0 王忠2019[21] CSM-TACE 45 2016—2017 回顾性 21/24 NA 5.43±1.68 NA 28/17/0 ①③④⑤ 7 cTACE 45 23/22 5.46±1.74 26/19/0 蒲长宇2020[22] CSM-TACE 60 2016—2018 回顾性 44/16 0/48/12 5.42±2.36 NA 34/26/0 ①③④⑤ 7 cTACE 60 46/14 0/45/15 5.32±1.81 36/24/0 刘娟芳2019[23] CSM-TACE 31 2016—2017 回顾性 NA NA NA NA NA ① 7 cTACE 40 李富永2020[24] CSM-TACE 38 2016—2018 回顾性 29/9 NA 4.7±1.2 NA 17/21/0 ① 7 cTACE 38 25/13 5.1±1.2 12/26/0 陈刚2017[25] CSM-TACE 22 2015—2016 回顾性 19/3 5/9/8 NA 7/15 15/7/0 ③④⑤ 7 cTACE 20 17/3 2/12/6 5/15 17/3/0 注: PVTT, 门静脉癌栓; ①总生存率; ②无进展生存率; ③发热率; ④腹痛; ⑤呕吐; ⑥腹水; ⑦骨髓抑制; ⑧粒细胞减少。 -
[1] SINGAL AG, LAMPERTICO P, NAHON P. Epidemiology and surveillance for hepatocellular carcinoma: New trends[J]. J Hepatol, 2020, 72(2): 250-261. DOI: 10.1016/j.jhep.2019.08.025. [2] ZHOU M, WANG H, ZENG X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2019, 394(10204): 1145-1158. DOI: 10.1016/S0140-6736(19)30427-1. [3] Chinese College of Interventionalists, Chinese Medical Doctor Association. Chinese clinical practice guidelines for transarterial chemoembolization of hepatocellular carcinoma[J]. J Intervent Radiol, 2018, 27(12): 1117-1126. DOI: 10.3969/j.issn.1008-794X.2018.12.001.中国医师协会介入医师分会. 中国肝细胞癌经动脉化疗栓塞治疗(TACE)临床实践指南[J]. 介入放射学杂志, 2018, 27(12): 1117-1126. DOI: 10.3969/j.issn.1008-794X.2018.12.001. [4] de BAERE T, ARAI Y, LENCIONI R, et al. Treatment of liver tumors with lipiodol TACE: Technical recommendations from experts opinion[J]. Cardiovasc Intervent Radiol, 2016, 39(3): 334-343. DOI: 10.1007/s00270-015-1208-y. [5] PESAPANE F, NEZAMI N, PATELLA F, et al. New concepts in embolotherapy of HCC[J]. Med Oncol, 2017, 34(4): 58. DOI: 10.1007/s12032-017-0917-2. [6] LEE EW, KHAN S. Recent advances in transarterial embolotherapies in the treatment of hepatocellular carcinoma[J]. Clin Mol Hepatol, 2017, 23(4): 265-272. DOI: 10.3350/cmh.2017.0111. [7] NAMUR J, CITRON SJ, SELLERS MT, et al. Embolization of hepatocellular carcinoma with drug-eluting beads: Doxorubicin tissue concentration and distribution in patient liver explants[J]. J Hepatol, 2011, 55(6): 1332-1338. DOI: 10.1016/j.jhep.2011.03.024. [8] ZHOU GH, HAN J, SUN JH, et al. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheresⓇ beads in Chinese hepatocellular carcinoma patients[J]. BMC Cancer, 2018, 18(1): 644. DOI: 10.1186/s12885-018-4566-4. [9] WU B, ZHOU J, LING G, et al. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: A short-term efficacy and safety study[J]. World J Surg Oncol, 2018, 16(1): 69. DOI: 10.1186/s12957-018-1368-8. [10] HUANG WH, FENG GS. Clinical analysis of polyvinyl alcohol callispheres in interventional embolization therapy for primary hepatocellular carcinoma[J]. J Pract Oncol, 2020, 35(3): 260-264. DOI: 10.13267/j.cnki.syzlzz.2020.03.014.黄文浩, 冯广森. 聚乙烯醇载药微球介入栓塞治疗原发性肝癌的临床分析[J]. 实用肿瘤杂志, 2020, 35(3): 260-264. DOI: 10.13267/j.cnki.syzlzz.2020.03.014. [11] PARMAR MK, TORRI V, STEWART L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints[J]. Stat Med, 1998, 17(24): 2815-2834. DOI: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [12] JADAD AR, MOORE RA, CARROLL D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?[J]. Control Clin Trials, 1996, 17(1): 1-12. DOI: 10.1016/0197-2456(95)00134-4. [13] MA Y, ZHAO C, ZHAO H, et al. Comparison of treatment efficacy and safety between drug-eluting bead transarterial chemoembolization with CalliSpheresⓇ microspheres and conventional transarterial chemoembolization as first-line treatment in hepatocellular carcinoma patients[J]. Am J Transl Res, 2019, 11(12): 7456-7470. [14] ZHANG ZS, LI HZ, MA C, et al. Conventional versus drug-eluting beads chemoembolization for infiltrative hepatocellular carcinoma: A comparison of efficacy and safety[J]. BMC Cancer, 2019, 19(1): 1162. DOI: 10.1186/s12885-019-6386-6. [15] XIAO YD, MA C, ZHANG ZS, et al. Safety and efficacy assessment of transarterial chemoembolization using drug-eluting beads in patients with hepatocellular carcinoma and arterioportal shunt: A single-center experience[J]. Cancer Manag Res, 2019, 11: 1551-1557. DOI: 10.2147/CMAR.S193948. [16] XIANG H, LONG L, YAO Y, et al. CalliSpheres drug-eluting bead transcatheter arterial chemoembolization presents with better efficacy and equal safety compared to conventional TACE in treating patients with hepatocellular carcinoma[J]. Technol Cancer Res Treat, 2019, 18: 1533033819830751. DOI: 10.1177/1533033819830751. [17] LI H, WU F, DUAN M, et al. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: A comparison of efficacy and safety[J]. Medicine (Baltimore), 2019, 98(21): e15314. DOI: 10.1097/MD.0000000000015314. [18] DUAN XH, JU SG, HAN XW, et al. Arsenic trioxide-eluting Callispheres beads is more effective and equally tolerant compared with arsenic trioxide/lipiodol emulsion in the transcatheter arterial chemoembolization treatment for unresectable hepatocellular carcinoma patients[J]. Eur Rev Med Pharmacol Sci, 2020, 24(3): 1468-1480. DOI: 10.26355/eurrev_202002_20206. [19] LIANG B, XIANG H, MA C, et al. Comparison of chemoembolization with CalliSpheres(Ⓡ) microspheres and conventional chemoembolization in the treatment of hepatocellular carcinoma: A multicenter retrospective study[J]. Cancer Manag Res, 2020, 12: 941-956. DOI: 10.2147/CMAR.S187203. [20] ZHOU SJ, WU BL, ZHOU J, et al. Short-term efficacy and safety of drug-eluting beads transarterial chemoembolization in the treatment of advanced primary hepatocellular carcinoma[J]. Med J Wuhan Univ, 2019, 40(5): 742-746. DOI: 10.14188/j.1671-8852.2018.1170.周素军, 吴宝林, 周军, 等. 药物缓释微球肝动脉化疗栓塞术治疗中晚期原发性肝癌的近期疗效及安全性分析[J]. 武汉大学学报(医学版), 2019, 40(5): 742-746. DOI: 10.14188/j.1671-8852.2018.1170. [21] WANG Z, LIU QY, YANG W, et al. Clinical value of transarterial chemoembolization with CalliSpheres drug-eluting beads manufactured in China in treatment of unresectable liver cancer[J]. Chin Hepatol, 2019, 24(7): 767-770. DOI: 10.3969/j.issn.1008-1704.2019.07.014.王忠, 刘启榆, 杨伟, 等. 国产CalliSpheres载药微球DEB-TACE治疗无法手术肝癌的临床价值[J]. 肝脏, 2019, 24(7): 767-770. DOI: 10.3969/j.issn.1008-1704.2019.07.014. [22] PU CY, CHEN Q. Application of drug-eluting beads in transarterial chemoembolization for advanced liver cancer and its effect on immune function[J]. Chin Hepatol, 2020, 25(1): 42-46. DOI: 10.3969/j.issn.1008-1704.2020.01.015.蒲长宇, 陈琦. 载药微球用于中晚期肝癌TACE术对肝功能及免疫功能的影响[J]. 肝脏, 2020, 25(1): 42-46. DOI: 10.3969/j.issn.1008-1704.2020.01.015. [23] LIU JF, DUAN XH, REN JZ, et al. Comparative effect of CalliSpheres drug loading microspheres and lipiodol transarterial chemoembolization in the treatment of huge primary liver cancer[J]. Chin J Hepatol, 2019, 27(6): 460-462. DOI: 10.3760/cma.j.issn.1007-3418.2019.06.014.刘娟芳, 段旭华, 任建庄, 等. CalliSpheres载药微球与碘油经导管动脉化疗栓塞治疗原发性巨块型肝癌的疗效对比[J]. 中华肝脏病杂志, 2019, 27(6): 460-462. DOI: 10.3760/cma.j.issn.1007-3418.2019.06.014. [24] LI FY, SUN YM, SHI MB. Value of drug-eluting beads versus ultra-fluid lipiodol in transarterial chemoembolization for liver cancer[J]. Mod Digest Interv, 2020, 25(5): 649-652. DOI: 10.3969/j.issn.1672-2159.2020.05.022.李富永, 孙玉敏, 石明波. 载药微球及超液化碘油用于肝癌肝动脉栓塞术的价值比较[J]. 现代消化及介入诊疗, 2020, 25(5): 649-652. DOI: 10.3969/j.issn.1672-2159.2020.05.022. [25] CHEN G, ZHANG D, YING YC, et al. Clinical investigation on transarterial chemoembolization with indigenous drug-eluting beads in treatment of unresectable hepatocellular carcinoma[J]. J Zhejiang Univ(Med Sci), 2017, 46(1): 44-51. DOI: 10.3785/j.issn.1008-9292.2017.02.07.陈刚, 张鼎, 应亚草, 等. 国产载药微球经动脉化疗栓塞治疗不可切除原发性肝癌的临床研究[J]. 浙江大学学报(医学版), 2017, 46(1): 44-51. DOI: 10.3785/j.issn.1008-9292.2017.02.07. [26] HA Y, LEE JB, SHIM JH, et al. Validation and reappraisal of the assessment for retreatment with transarterial chemoembolization score for unresectable non-metastatic hepatocellular carcinoma in a hepatitis B virus-endemic region[J]. Eur Radiol, 2016, 26(10): 3510-3518. DOI: 10.1007/s00330-015-4185-2. [27] GOLFIERI R, GIAMPALMA E, RENZULLI M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma[J]. Br J Cancer, 2014, 111(2): 255-264. DOI: 10.1038/bjc.2014.199. [28] MEMON K, KULIK L, LEWANDOWSKI RJ, et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times[J]. Gastroenterology, 2011, 141(2): 526-535. DOI: 10.1053/j.gastro.2011.04.054. [29] YIN WL, LIAN J, XIAO SX, et al. Clinical effect of drug-eluting beads and conventional transcatheter arterial chemoembolization in treatment of unre-sectable liver cancer: A meta-analysis[J]. J Clin Hepatol, 2019, 35(6): 1270-1275. DOI: 10.3969/j.issn.1001-5256.2019.06.018.尹伟利, 连佳, 肖时湘, 等. 载药微球与传统碘化油经肝动脉化疗栓塞术治疗不可切除肝癌效果比较的Meta分析[J]. 临床肝胆病杂志, 2019, 35(6): 1270-1275. DOI: 10.3969/j.issn.1001-5256.2019.06.018. [30] CHEN P, YUAN P, CHEN B, et al. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: A systematic review and meta-analysis[J]. Clin Res Hepatol Gastroenterol, 2017, 41(1): 75-85. DOI: 10.1016/j.clinre.2016.05.013. [31] JIANG S, LI GJ, ZHOU ZQ, et al. Interventional chemoembolization with CalliSpheres-loaded microspheres for the treatment of advanced hepatocellular carcinoma[J/CD]. Chin J Inter Rad(Electronic Edition), 2017, 5(3): 174-178. DOI: 10.3877/cma.j.issn.2095-5782.2017.03.013.姜松, 李桂杰, 周祝谦, 等. CalliSpheres载药栓塞微球治疗中晚期肝癌临床效果评价[J/CD]. 中华介入放射学电子杂志, 2017, 5(3): 174-178. DOI: 10.3877/cma.j.issn.2095-5782.2017.03.013. [32] XIE XC, ZHAO W, FAN HJ, et al. Safety and efficacy analysis of CalliSpheres drug-eluting beads TACE in the treatment of advanced liver cancer[J]. J Pract Radiol, 2020, 36(5): 792-795. DOI: 10.3969/j.issn.1002-1671.2020.05.026.谢璇丞, 赵卫, 范宏杰, 等. CalliSpheres载药微球动脉化疗栓塞术治疗中晚期肝癌的安全性及有效性分析[J]. 实用放射学杂志, 2020, 36(5): 792-795. DOI: 10.3969/j.issn.1002-1671.2020.05.026. [33] HONG K, KHWAJA A, LIAPI E, et al. New intra-arterial drug delivery system for the treatment of liver cancer: Preclinical assessment in a rabbit model of liver cancer[J]. Clin Cancer Res, 2006, 12(8): 2563-2567. DOI: 10.1158/1078-0432.CCR-05-2225. [34] TAYLOR RR, TANG Y, GONZALEZ MV, et al. Irinotecan drug eluting beads for use in chemoembolization: In vitro and in vivo evaluation of drug release properties[J]. Eur J Pharm Sci, 2007, 30(1): 7-14. DOI: 10.1016/j.ejps.2006.09.002. [35] LAMMER J, MALAGARI K, VOGL T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study[J]. Cardiovasc Intervent Radiol, 2010, 33(1): 41-52. DOI: 10.1007/s00270-009-9711-7. -



 PDF下载 ( 3201 KB)
PDF下载 ( 3201 KB)

 下载:
下载: