慢性乙型肝炎抗病毒新药的临床研究
DOI: 10.3969/j.issn.1001-5256.2021.05.003
利益冲突声明: 所有作者均声明不存在利益冲突。
作者贡献声明:张洪、丁艳华负责提出研究选题、撰写论文和修订论文;朱晓雪、买佳佳、陈红、周景、胡月、徐佳负责文献资料的检索和整理。
Clinical studies of new antiviral drugs for chronic hepatitis B
-
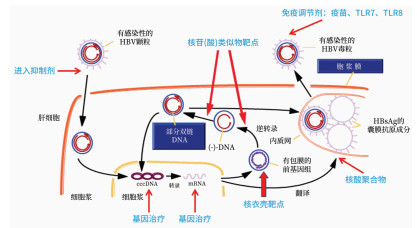
摘要: HBV感染是全球重大公共卫生问题。慢性乙型肝炎(CHB)临床治愈(亦称功能性治愈)是目前国内外最新CHB防治指南推荐的理想治疗目标。以直接抗病毒药物[如核苷(酸)类似物(NAs)]或免疫调节剂[如聚乙二醇化干扰素(PEG-IFN)α]序贯或联合治疗的优化方案治愈率都不高。CHB治疗药物的研发领域近年正处于快速发展阶段。综述了CHB抗病毒新药的临床研究,包括受试者的选择、研究设计、给药剂量、剂量爬坡、不良事件、疗效评价等关键环节。需要引入定量药理学知识来分析药物的体内暴露量与药效、不良反应的关系,以及纳入探索性指标进行分析。为抗HBV新药临床研发提供经验和新思路。Abstract: Hepatitis B virus (HBV) infection is a major global public health issue. Clinical cure (also known as functional cure) of chronic hepatitis B (CHB) is the ideal therapeutic goal recommended by the latest guidelines for the prevention and treatment of CHB in China and globally. Optimized treatment regimens with direct-acting antiviral agents [e.g., nucleos(t)ide analogues] or immunomodulators (e.g., pegylated interferon-α) sequentially or in combination tend to have low cure rates. Rapid development has been achieved in the research and development of drugs for the treatment of CHB. This article reviews the clinical study of new antiviral drugs for CHB, including the selection of subjects, study design, dosage, dose escalation, adverse events, and efficacy evaluation. It is necessary to introduce the knowledge of quantitative pharmacology to analyze the association of drug exposure in body with efficacy and adverse reactions, and exploratory indicators should be incorporated for comprehensive analysis. This review provides related experience and new ideas for the clinical research and development of new anti-HBV drugs.
-
Key words:
- Hepatitis B, Chronic /
- Clinical Trial /
- Drug Therapy
-
[1] Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648- 2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [2] SMOLDERS EJ, BURGER DM, FELD JJ, et al. Review article: Clinical pharmacology of current and investigational hepatitis B virus therapies[J]. Aliment Pharmacol Ther, 2020, 51(2): 231-243. DOI: 10.1111/apt.15581. [3] HUI CK, LEUNG N, YUEN ST, et al. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase[J]. Hepatology, 2007, 46(2): 395-401. DOI: 10.1002/hep.21724. [4] Center for Drug Evaluation, National Medical Products Administration. Technical guidelines for clinical trials of antiviral therapeutic drugs for chronic hepatitis B[EB/OL].(2018-02-09)[2021-03-12]. http://www.cde.org.cn/zdyz.do?method=largePage&id=90cd8637af74e7bb.国家药品监督管理局药品审评中心. 慢性乙型肝炎抗病毒治疗药物临床试验技术指导原则[EB/OL]. (2018-02-09)[2021-03-12]. http://www.cde.org.cn/zdyz.do?method=largePage&id=90cd8637af74e7bb. [5] SACKS LV, SHAMSUDDIN HH, YASINSKAYA YI, et al. Scientific and regulatory reasons for delay and denial of FDA approval of initial applications for new drugs, 2000-2012[J]. JAMA, 2014, 311(4): 378-384. DOI: 10.1001/jama.2013.282542. [6] US Food and drug Administration. Chronic hepatitis D virus infection: Developing drugs for treatment guidance for industry[EB/OL]. (2019-10-31)[2021-03-11]https://www.fda.gov/regulatory-information/search-fda-guidance-documents/chronic-hepatitis-d-virus-infection-developing-drugs-treatment-guidance-industry. [7] CORNBERG M, LOK AS, TERRAULT NA, et al. Guidance for design and endpoints of clinical trials in chronic hepatitis B-Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference[J]. Hepatology, 2020, 72(3): 539-557. doi: 10.1016/j.jhep.2019.11.003. [8] RAJORIYA N, COMBET C, ZOULIM F, et al. How viral genetic variants and genotypes influence disease and treatment outcome of chronic hepatitis B. Time for an individualised approach?[J]. J Hepatol, 2017, 67(6): 1281-1297. DOI: 10.1016/j.jhep.2017.07.011. [9] VERRIER ER, COLPITTS CC, SUREAU C, et al. Hepatitis B virus receptors and molecular drug targets[J]. Hepatol Int, 2016, 10(4): 567-573. DOI: 10.1007/s12072-016-9718-5. [10] URBAN S, BARTENSCHLAGER R, KUBITZ R, et al. Strategies to inhibit entry of HBV and HDV into hepatocytes[J]. Gastroenterology, 2014, 147(1): 48-64. DOI: 10.1053/j.gastro.2014.04.030. [11] WEDEMEYER H, SCHÖNEWEIS K, BOGOMOLOV PO, et al. GS-13-Final results of a multicenter, open-label phase 2 clinical trial (MYR203) to assess safety and efficacy of myrcludex B in cwith PEG-interferon Alpha 2a in patients with chronic HBV/HDV co-infection[J]. J Hepatol, 2019, 70(Suppl 1): e81. http://www.sciencedirect.com/science/article/pii/S0618827819301410 [12] ZHANG M, ZHANG J, TAN Y, et al. SAT452-efficacy and safety of GLS4/ritonavir combined with entecavir in HBeAg-positive patients with chronic hepatitis B: Interim results from phase 2b, multi-center study[J]. J Hepatol, 2020, 73: s878. [13] YUEN M, BERLIBA E, SUKEEPAISARNJAROEN W, et al. Safety, tolerability, pharmacokinetics (PK), and antiviral activity of the capsid inhibitor (CI) AB-506 in healthy subjects (HS) and chronic hepatitis B (CHB) subjects[J]. Hepatology, 2019, 70(6): 1492A-1493A. http://hub.hku.hk/handle/10722/289899 [14] BILLIOUD G, KRUSE RL, CARRILLO M, et al. In vivo reduction of hepatitis B virus antigenemia and viremia by antisense oligonucleotides[J]. J Hepatol, 2016, 64(4): 781-789. DOI: 10.1016/j.jhep.2015.11.032. [15] PAN J, TONG S, KANG L, et al. New anti-hepatitis B virus drugs under development and evaluation[J]. Curr Opin Infect Dis, 2016, 29(6): 632-638. DOI: 10.1097/QCO.0000000000000318. [16] YUEN M, LOCARNINI S, GIVEN B, et al. First clinical experience with RNA interference[RNAi]-based triple combination therapy in chronic hepatitis B (CHB): JNJ-73763989(JNJ-3989), JNJ-56136379(JNJ-6379) and a nucleos(t)ide analogue (NA)[J]. Hepatology, 2019, 70(6): 1489A. [17] MENG Z, LU M. RNA Interference-induced innate immunity, off-target effect, or immune adjuvant?[J]. Front Immunol, 2017, 8: 331. DOI: 10.3389/fimmu.2017.00331. [18] NOORDEEN F, VAILLANT A, JILBERT AR. Nucleic acid polymers prevent the establishment of duck hepatitis B virus infection in vivo[J]. Antimicrob Agents Chemother, 2013, 57(11): 5299-5306. DOI: 10.1128/AAC.01005-13. [19] VAILLANT A. Nucleic acid polymers: Broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection[J]. Antiviral Res, 2016, 133: 32-40. DOI: 10.1016/j.antiviral.2016.07.004. [20] GOHIL V, MISNER D, CHANDA S, et al. The S-antigen transport-inhibiting polymer (STOPS (TM)) ALG-010133[J]. Hepatology, 2020, 72: 508A. [21] BAZINET M, PÂNTEA V, PLACINTA G, et al. Safety and efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon alfa-2a in patients with chronic HBV infection naïve to nucleos(t)ide therapy[J]. Gastroenterology, 2020, 158(8): 2180-2194. DOI: 10.1053/j.gastro.2020.02.058. [22] BARNES E. Therapeutic vaccines in HBV: Lessons from HCV[J]. Med Microbiol Immunol, 2015, 204(1): 79-86. DOI: 10.1007/s00430-014-0376-8. [23] ZHANG E, KOSINSKA A, LU M, et al. Current status of immunomodulatory therapy in chronic hepatitis B, fifty years after discovery of the virus: Search for the "magic bullet" to kill cccDNA[J]. Antiviral Res, 2015, 123: 193-203. DOI: 10.1016/j.antiviral.2015.10.009. [24] HERSCHKE F, LI C, de CREUS A, et al. Antiviral activity of JNJ-4964 (AL-034/TQ-A3334), a selective toll-like receptor 7 agonist, in AAV/HBV mice after oral administration for 12 weeks[J]. J Hepatol, 2019, 70(1): e49-e50. http://www.sciencedirect.com/science/article/pii/S061882781930088X [25] GANE E, DUNBAR PR, BROOKS A, et al. AS071-efficacy and safety of 24 weeks treatment with oral TLR8 agonist, selgantolimod, in virally-suppressed adult patients with chronic hepatitis B: A phase 2 study[J]. J Hepatol, 2020, 73: s52. [26] BAUM P, ODEGARD V. ASGR1-TLR8. An ASGR1-directed TLR8 immunotac(TM) therapeutic, is a potent myeloid cell agonist with liver-localized activity for the treatment of chronic HBV[J]. Hepatology, 2020, 72: 503A. DOI: 10.1002/hep.31039 -



 PDF下载 ( 2154 KB)
PDF下载 ( 2154 KB)

 下载:
下载:


